Invertebrate Anatomy OnLine
Velella velella ©
By-the-WindSailor
30may2007
Copyright 2007 by
Richard Fox
Lander University
Preface
This is an exercise from Invertebrate Anatomy OnLine , an Internet laboratory manual for courses in Invertebrate Zoology. Additional exercises can be accessed by clicking on the link. A glossary and chapters on supplies and laboratory techniques are also available through this link. Terminology and phylogeny used in these exercises correspond to usage in the textbook by Ruppert, Fox, and Barnes (2004). Hyphenated figure callouts refer to figures in the textbook. Callouts that are not hyphenated refer to figures embedded in the exercise. The glossary includes terms from this textbook as well as the laboratory exercises.
Systematics
Cnidaria P, Hydrozoa C, “A-Form”, Anthothecatae O, Capitata sO, Chondrophoridae F,
Cnidaria P
The cnidarian body consists of a central blind sac, the coelenteron (= gastrovascular cavity), enclosed by a body wall comprising two epithelia, the outer epidermis and the inner gastrodermis (Fig 7-1, 7-2*). A gelatinous connective tissue layer, the mesoglea, lies between the two epithelia. The mouth opens at one end of the coelenteron and marks the oral end. The mouth is at the tip of a process, the manubrium that elevates it above the oral surface. The opposite pole is the aboral end. The imaginary line connecting the oral and aboral poles is the axis of symmetry around which the radial symmetry of the body is organized. The mouth is usually surrounded by one or more circles of tentacles.
The defining cnidarian feature is, of course, their possession of stinging cells, or cnidocytes (Fig 7-8). Characteristic of the epidermis, they are also sometimes found in the gastrodermis. Cnidocytes contain an explosive organelle, the cnida, which, upon proper stimulation, inverts and ejects a slender, often barbed and toxic thread in the direction of prey or predator (Fig 7-9). Three types of cnidae are found in cnidarians (Fig 7-10). Nematocysts (in nematocytes), spirocysts (in spirocytes), and ptychocysts (in ptychocytes). All toxic cnidae are nematocysts whereas spirocysts are sticky, and the everted tubules of ptychocysts are used for constructing feltlike tubes. Most cnidae are nematocysts and these are present in all three higher cnidarian taxa. Spirocysts and ptychocysts are found only in Anthozoa.
The basic body plan described above can be manifest as a swimming medusa or attached polyp. In some taxa only one generation is present whereas in others both are found. A life cycle featuring alternation of sexual, swimming medusae with benthic asexual polyps is typical of many cnidarians.
All cnidarians are carnivores feeding on live prey which they usually capture using tentacles armed with cnidocytes. Digestion occurs in the coelenteron which is typically equipped with ciliated canals for distribution of partly digested food. Cnidarians are ammonotelic and diffusion across the body and tentacle surface eliminated the ammonia from the body. Gas exchange is across the general body surface. The nervous system is a plexus of basiepithelial neurons serving sensory and motor systems (Fig 7-6). Most cnidarians are gonochoric. The life cycle typically includes a planula larva. Cnidarians are chiefly marine but the well-known Hydra is an exception.
Medusozoa
Medusozoa comprises those cnidarians whose life cycle includes a medusa generation that alternates with a polyp generation (Fig 7-75B). Symmetry is radial and tetramerous. Nematocysts are the only type of cnidocyte present. Included taxa are Scyphozoa (jellyfishes) and Hydrozoa (hydroids, Hydra, Portuguese men of war, etc).
Hydrozoa C
Hydrozoa is a diverse taxon of about 3000 species of mostly marine cnidarians. The life cycle usually includes both polyp and medusa generations (Fig 7-65A) but may be entirely polyp (Fig 7-65B) or entirely medusa (Fig 7-65C). Polyps typically are colonial and medusae usually solitary. Some form colonies of combinations of polyps and medusae. The few freshwater cnidarians, such as Hydra, Vallentinia, and Craspedacusta, are hydrozoans.
Hydrozoan polyps are usually small, about 1 mm in length, and colonial. Hydromedusae are also small, at least in comparison with scyphomedusae, and are usually less than 1 cm in diameter. Hydromedusae are further distinguished from scyphomedusae by possession of a velum, a circumferential shelf of tissue that encircles the subumbrellar concavity and functions as an adjustable diaphragm to create a pulse of water for swimming. Medusae are tetramerously symmetrical as are scyphomedusae and four radial canals and four tentacles are usually present.
Cnidocytes are found only in the epidermis and germ cells arise in the gastrodermis but may, or may not, migrate to the epidermis to form gonads and mature into gametes. Nematocysts are the only cnida. Gonads may be on the manubrium or on the radial canals. Gametes, even if gastrodermal, are released directly to the surrounding water, never, in contrast with scyphomedusae, into the coelenteron.
“A-forms”
A-form hydrozoans have athecate polyps and tall medusae with manubrial gonads and ocelli.
Anthoathecatae O
Polyps athecate.
Capitata sO
Tentacles are capitate, terminating in swellings with abundant nematocytes.
Natural History
Velella, the by-the-wind sailor, is a colonial hydrozoan found floating in the surface community (pleuston) of temperate and tropical seas. It is small rhomboid, usually reaching only about 6 cm in length and 3 cm in width. It floats at the sea surface, with part of the colony in air and part submersed, held in position by a chitinous, air-filled float (Fig 1). A stiff triangular sail sits atop the float. At sea Vellella occurs in rafts. In the company of other pleustonic animals, such as the snail Janthina, another chondrophoran, Porpita, and the nudibranch Glaucus, Velella may be blown ashore and stranded on beaches after storms. Population densities may be enormous. There is a record of a raft of Velella in the Atlantic 260 km long. Windrows 1 km in length and 50 cm high have been reported on beaches.
Figure 1. Velella viewed from the side. Hydrozoa94.gif
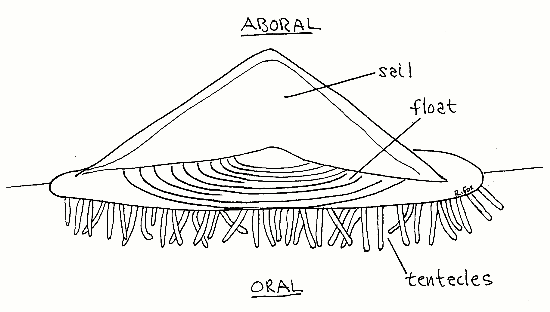
Velella is a passive sailor that travels where wind and currents take it. Individuals have sails angled oblique to the long axis (Fig 2) but are either right or left-handed. In half the population the sail is rotated to the right of the longitudinal axis and in the other half it is to the left. The wind thus will blow half of a mixed population in one direction and the other half in another, thereby sorting it so that the entire population is not blown ashore, at least not by moderate winds. Strong winds blow all individuals in the same direction. Wind tunnel studies have shown that the design of the sail maximizes stability and seaworthiness at the expense of performance.
Velella is a zooplanktivore feeding on fish larvae, fish eggs, copepods, crustacean larvae, and appendicularians. It has gastrodermal zooxanthellae, which presumably contribute to its nutrition. Velella is itself consumed by other members of the pleuston, namely the snail, Janthina, and the nudibranch, Glaucus.
In life Velella is the beautiful deep blue of the open ocean but preserved it is colorless. Janthina janthina, Porpita porpita, and Glaucus atlanticus share this blue color. Velella, Porpita, Glaucus, and Janthina all have gas-filled bladders of some type and use them to maintain positive buoyancy and assure their position in the pleuston.
Velella’s cnidocytes, although equipped with toxin, are generally incapable of penetrating human skin although some observers have reported irritation of sensitive areas such as the eyes or tongue.
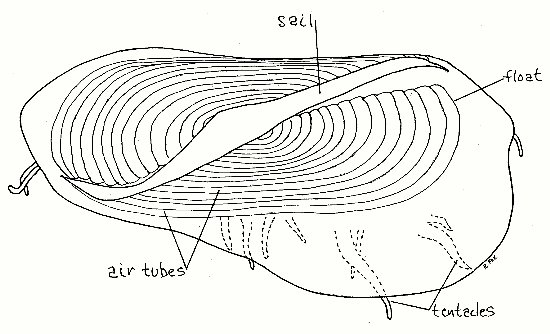
Figure 2. Aboral view of a 3.5 cm Velella stranded at Vero Beach, Florida, December 2006. The sail is canted to the left as were those of all 35 specimens collected at the same time from this beach.
Laboratory Specimens
This exercise was written using preserved specimens collected by Drs. Edward Ruppert and Jennifer Frick-Ruppert at Vero Beach, Florida in December 2006. Preserved specimens are available from Wards Natural Science (www.wardsci.com). The anatomy of Porpita closely resembles that of Velella and the following description applies as well to Porpita as to Velella except that Porpita is circular instead of rhomboidal and Porpita lacks a sail. Either could be used for this study.
Anatomy
Velella (and Porpita) is a colony of polyps consisting of a central axial feeding polyp surrounded by a ring of reproductive polyps and a ring of tentacles (which may also be polyps). Some authorities consider Velella and Porpita to be single individuals rather than colonies but, since they consists of multiple polyps, they are here considered to be colonial.
The upper, or aboral, surface of Velella floats above the water surface and consists of a sail and flotation device (Fig 1). The lower surface, which in life is submersed below the water surface, is the oral surface. It bears numerous fingerlike projections that are the tentacles and polyps. Between the upper float and the dangling polyps is a large central mass of coenosarc consisting mostly of mesoglea penetrated by tubular, gastrodermal extensions of the coelenteron, the solenia. Layered between upper and lower portions of the central mass is a layer of ectodermal tissue filled with cnidoblasts and known as the central organ (Fig 3). Cnidoblasts originate here and migrate to the tentacles and polyps to become stinging cells (cnidocytes). The mouths of the polyps face downward, or orally, and away from the sea surface.
Begin your study with a preserved specimen in a dish with about 2 mm of tap water in the bottom. The specimen should rest on the bottom of the dish so it does not capsize while you are trying to look at its upper surface. (Preserved specimens in tap water will not float naturally and will tend to flop over on their side or turn upside down if the water is deep enough.) Moisten the specimen periodically. (If your specimen is living, use seawater or isotonic magnesium chloride.) Use the dissecting microscope as necessary.
Arrange the specimen so the sail is uppermost, which is its position in life. The body of the colony is a disc roughly rhomboidal in outline but with one end a little larger than the other. This upper surface is the aboral surface. The aboral surface is dominated by a large, oval, chitinous float, or pneumatophore (Fig 2). The float is composed of numerous concentric, air-filled, chitinous air tubes. The air tubes are easy to see when filled with air, as they usually are, but if water or preservative has filled the tubes they become harder to see. Each air tube communicates with the atmosphere via two small air pores, or pneumostomes, but these are difficult to demonstrate (Fig 3). Arising from the air tubes is a system of minute, branching, air-filled tracheae extending into the coenosarc (Fig 3, 7-67). Tracheae, being diverticula of the chitinous walls of the pneumatophore, are themselves strengthened and supported by chitinous rings, like those of insect tracheae (and functionally similar to the cartilaginous rings that support vertebrate tracheae.)
The rigid sail is a conspicuous, triangular, chitinous membrane extending upward from the float. The long axis of the sail is oblique to the long axis of the float being shifted either to the right or left of that axis. A thin flange or fold of tissue extending to the side from each end of the float imparts an asymmetry or rhomboidal shape to the body when viewed from above. The perfect oval of the float thus becomes an irregular rhomboid (Fig 2).
Add enough water to the dish to allow the specimen to turn over and float with its oral surface uppermost. Oral to the float is a mass of mesoglea, known as the coenosarc, which is penetrated by tubular solenia arising from the coelenteron (Fig 3). The coenosarc is olive brown due to the presence of zooxanthellae in gastrodermal cells lining the solenia.
In the center of the aboral surface, under the coenosarc and often obscured by the surrounding polyps, is a single large gastrozooid (Fig 3). The gastrozooid hangs below the central mass. In preserved specimens the gastrozooid is contracted and thimble or bell-shaped. The mouth is at the apex of the thimble but is tightly contracted in preserved specimens and difficult to demonstrate. Probe the apex with a microneedle to demonstrate the mouth. The mouth opens into a buccal cavity in the interior of the gastrozooid. The buccal cavity is continuous with the stomach aboral to it. Viewed from the oral surface the stomach appears as an elongate, spindle-shaped chamber whose long axis coincides with that of the colony. Eight radial canals extend from the stomach to the periphery of the float to join a ring canal. The coelenteron consists of the buccal cavity, stomach, radial canals, a ring canal, and the abundant solenia (Fig 3).
Figure 3. Longitudinal section of Velella. Most gonozooids and tentacles have been omitted for clarity. Most solenia have been truncated. (Redrawn from Delage & Hérouard, 1901). Hydrozoa96.gif
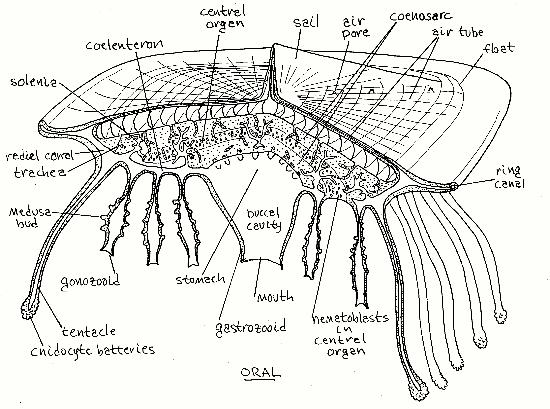
The gastrozooid and stomach are surrounded by a ring of tangled (in preserved specimens), elongate gonozooids. Each gonozooid bears numerous thimble-shaped medusae buds. Each gonozooid has a terminal (oral) mouth but they are contracted in preserved specimens and difficult to find. The lumina of the gonozooids connect with the coelenteron.
Remove a gonozooid from the colony and make a wetmount with it. Examine it at 100X to find medusae buds. At 400X look for nematocysts. They are clustered in cnidocyte batteries on the medusa buds. Look for nematocysts on parts of the gonozooid other than the buds.
Arising on the oral surface under the outer margin of the float is a single row of mouthless digitiform tentacles, sometimes considered to be dactylozooids, a type of polyp (Fig 3). The tentacles are used to sting prey into submission or for defense, although they are ineffective against Janthina and Glaucus.
With fine scissors snip about 3 mm from the end of a tentacle and make a wetmount for examination with 400X. Adjust the light carefully. Find circular and longitudinal muscles. Abundant oval nematocysts will be evident and you should be able to find some that have discharged, revealing their thread and strong basal barbs. Find some undischarged nematocysts with the thread still coiled inside.
Medusae
Anthomedusae are produced asexually from medusa buds on the gonozooids. The tiny medusae are less than 3 mm in height. Medusae have two pairs of capitate tentacles and four gonads on the manubrium. They are gonochoric and reproduce sexually, producing eggs and sperm by meiosis. Medusae do not remain in the pleuston and are found as deep as 2000 meters below the surface.
Little is known of development but the youngest embryos are found in deep water. Development lasts about six weeks and as it proceeds accumulation of oil droplets causes the embryos to migrate gradually toward the surface.
References
Delage Y, Hérouard E. 1901. Traité de Zoologie Concrète Tome II, partie 2. Les Coelentérés. 848pp. (Chondrophores covered on pp 253-264.)
Francis L. 1991. Sailing downwind: aerodynamic performance of the Velella sail. J. Experimental Biology 158:117-132.
Hickson SJ. 1909. Coelenterata and Ctenophora, pp.243-424 in Harmer SF, Shipley AE (eds), Cambridge Natural History, vol 1. MacMillan, London. 671 pp.
Kaestner A. 1967. Invertebrate zoology, vol.1. Interscience, New York. 597pp.
Larson R. 1980. The medusa of Velella velella (Linnaeus, 1758) 0(Hydrozoa, Chondrophorae). J Plankton Research 2:183-186.
Ruppert EE, Fox RS, Barnes RB. 2004. Invertebrate Zoology, A functional evolutionary approach, 7 th ed. Brooks Cole Thomson, Belmont CA. 963 pp.
Supplies
living or preserved Velella
microdissecting tools
slides, coverslips
dissecting microscope
compound microscope