Invertebrate Anatomy
OnLine
Triops
longicaudatus
©
Tadpole Shrimp
19jun2006
Copyright 2001 by
Richard Fox
Lander University
Preface
This is one of many
exercises available from
Invertebrate Anatomy OnLine
,
an Internet laboratory manual for
courses in Invertebrate Zoology. Additional exercises can be
accessed by clicking on the links to the left. A glossary and
chapters on supplies and laboratory techniques are also
available. Terminology and phylogeny used in these exercises
correspond to usage in the Invertebrate Zoology textbook by Ruppert,
Fox, and Barnes (2004). Hyphenated figure callouts refer to figures
in the textbook. Callouts that are not hyphenated refer to figures
embedded in the exercise. The glossary includes terms from this
textbook as well as the laboratory exercises.
Systematics
Arthropoda P, Mandibulata, Crustacea sP,
Eucrustacea, Thoracopoda, Phyllopodomorpha, Phyllopoda, Notostraca
O, Triopsidae F (Fig 16-15, 19-18, 19-90)
Arthropoda P
Arthropoda, by far the largest and most diverse animal
taxon, includes chelicerates, insects, myriapods, and crustaceans as
well as many extinct taxa such as Trilobitomorpha. The segmented
body primitively bears a pair of jointed appendages on each
segment. The epidermis secretes a complex cuticular exoskeleton
which must be molted to permit increase in size. Extant arthropods
exhibit regional specialization in the structure and function of
segments and appendages but the ancestor probably had similar
appendages on all segments. The body is typically divided into a
head and trunk, of which the trunk is often further divided into
thorax and abdomen.
The gut consists of foregut, midgut, and hindgut and
extends the length of the body from anterior mouth to posterior
anus. Foregut and hindgut are epidermal invaginations, being derived
from the embryonic stomodeum and proctodeum respectively, and are
lined by cuticle, as are all epidermal surfaces of arthropods. The
midgut is endodermal and is responsible for most enzyme secretion,
hydrolysis, and absorption.
The coelom is reduced to small spaces associated with
the gonads and kidney. The functional body cavity is a spacious
hemocoel divided by a horizontal diaphragm into a dorsal pericardial
sinus and a much larger perivisceral sinus. Sometimes there is a
small ventral perineural sinus surrounding the ventral nerve cord.
The hemal system includes a dorsal, contractile,
tubular, ostiate heart that pumps blood to the hemocoel. Excretory
organs vary with taxon and include Malpighian tubules, saccate
nephridia, and nephrocytes. Respiratory organs also vary with taxon
and include many types of gills, book lungs, and tracheae.
The nervous system consists of a dorsal, anterior brain
of two or three pairs of ganglia, circumenteric connectives, and a
paired ventral nerve cord with segmental ganglia and segmental
peripheral nerves. Various degrees of condensation and cephalization
are found in different taxa.
Development is derived with centrolecithal eggs and
superficial cleavage. There is frequently a larva although
development is direct in many. Juveniles pass through a series of
instars separated by molts until reaching the adult size and
reproductive condition. At this time molting and growth may cease or
continue, depending on taxon.
Mandibulata
Mandibulata is the sister taxon of Chelicerata and
in contrast has antennae on the first head segment, mandibles on the
third, and maxillae on the fourth. The brain is a syncerebrum with
three pairs of ganglia rather than the two of chelicerates. The
ancestral mandibulate probably had biramous appendages and a
J-shaped gut, posterior-facing mouth, and a ventral food groove. The
two highest level mandibulate taxa are Crustacea and Tracheata.
Crustacea sP
Crustacea is the sister taxon of Tracheata and is
different in having antennae on the second head segment resulting in
a total of 2 pairs, which is unique. The original crustacean
appendages were biramous but uniramous limbs are common in derived
taxa. The original tagmata were head but this has been replaced by
head, thorax, and abdomen or cephalothorax and abdomen in many taxa.
Excretion is via one, sometimes two, pairs of saccate nephridia and
respiration is accomplished by a wide variety of gills, sometimes by
the body surface. The nauplius is the earliest hatching stage and
the naupliar eye consists of three or four median ocelli.
Eucrustacea
Eucrustacea includes all Recent crustaceans except
the remipedes. The taxon is characterized by a primary tagmosis
consisting of heat, thorax, and abdomen although the derived
condition of cephalothorax and abdomen is more common. Eight is the
maximum number of thoracic segments.
Thoracopoda
In the ancestral thoracopod the thoracic
appendages were turgor appendages used for suspension feeding in
conjunction with a ventral food groove. Such appendages and feeding
persist in several Recent taxa but have been modified in many
others.
Phyllopodomorpha
The compound eyes are stalked primitively although
derived sessile eyes occur in many taxa.
Phyllopoda
Phyllopoda consists of about 800 species in four higher
taxa; the “large phyllopodans” consisting of Notostraca,
Laevicaudata, and Spinicaudata and Cladocera, which are the “small
phyllopodans”. Trunk appendages are phyllopods and a large carapace
encloses much or all of the body. Large phyllopodans typically
inhabit relictual habits where fishes are absent but Cladocerans
show no such restrictions. Tagmata are a head, thorax, and reduced
abdomen. The abdomen lacks appendages but has a posterior caudal
furca on the telson. A ventral food groove is usually present and
employed in feeding. A so-called dorsal organ is present on the
dorsal midline of the posterior head.
Notostraca O
Notostracans are tadpole shrimps, of which only 10
species are known worldwide. They inhabit quiet, fishless, usually
temporary, freshwaters where they crawl over the bottom or swim in
the water. They use the anterior trunk appendages for both types of
locomotion as well as for feeding. Tadpole shrimps are deposit
feeders and predators. They are sometimes abundant in rice
fields. Notostracans differ from anostracans primarily in having a
carapace (noto=back, ostrac=shell), sessile compound eyes, and
appendages posterior to the genital segments. The trunk is composed
of about 40 segments and is divided into a large thorax and a small
abdomen.
Laboratory Specimens
Viable tadpole shrimp eggs are available from Ward's
Natural Science Co. (see Supplies chapter). This company collects
detritus, including eggs, from the bottom of temporary ponds in Utah
and ships it under the name "living fossils". The eggs are easily
hatched and the shrimp can be reared to maturity in the
laboratory. It is thus possible to see living tadpole shrimps in any
laboratory, an opportunity that few biologists, especially those
living in the eastern United States, would ever have. The eggs
provided are those of Triops longicaudatus.
All notostracans are similar and this exercise can be
used for any species. All North American species are western (or
boreal) and belong to the genera Triops (= Apus)
or Lepidurus. The exercise emphasizes external anatomy. The
internal organs resemble those of anostracans such as brine
shrimp. As usual, living material is preferable to preserved but
either is acceptable, especially for study of external anatomy.
Behavior
Examine a living tadpole shrimp in an 8-cm culture dish
of pondwater. Place the dish on the stage of your dissecting
microscope, with the substage light off, and watch it
swim. Notostracans, like most aquatic animals but unlike
anostracans, exhibit a dorsal light response,
swimming with the dorsum facing toward the light source. In nature
the normal swimming posture is right side up (with the dorsum
up). In laboratory situations individuals can be induced to swim
upside down (with the dorsum down), if a light is placed beneath
them.
>1a. On the stage of the dissecting
microscope watch a tadpole shrimp swim in a glass dish with overhead
(incident) illumination and note the nature of the light
response. It is dorsal or ventral? Which surface is usually up? Turn
the substage lamp on and observe the response. Does the orientation
of the animal change? Now which surface is usually up? <
External Anatomy
Tagmata
Study the external anatomy of your specimen. If it is
alive place a drop of chloroform in the dish and wait it to become
inactive.
Look first at the dorsal surface. The body
consists of a head and trunk and
is mostly covered by the large, dorsal carapace
(Fig 1, 19-13). Little of the body is visible dorsally. Turn the
animal over and look at the ventral surface.
Figure 1. Dorsal view of a tadpole shrimp, Triops
longicaudatus, reared from sediments from a temporary pond in
Utah. Notostraca1L.gif
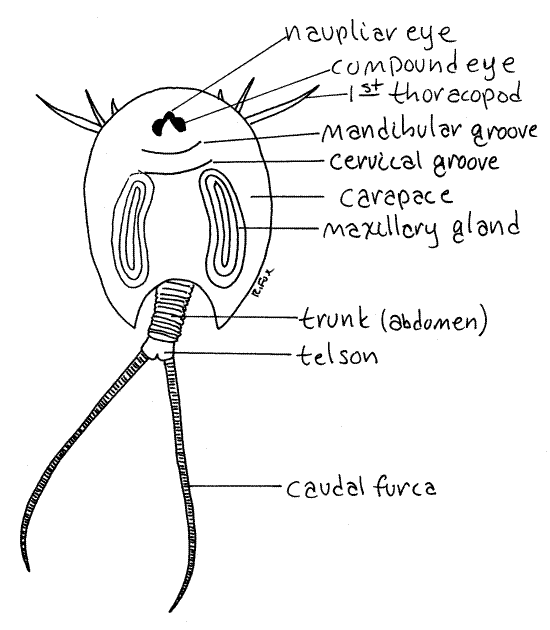
The head is typical of crustaceans and
is composed of five fused segments but there is a tendency to
reduction or loss of head appendages. The long trunk is not
distinctly divided into thorax and abdomen. Most of the trunk
segments bear appendages.
As you study the animal try to decide where you think
the thorax stops and the abdomen begins. The issue is disputed. The
first 11 trunk segments each bear a pair of appendages. These are
followed by a region of fused segments each of which bears up to six
pairs of appendages. Finally the trunk ends with a region of
segments with no appendages. Some biologists consider the
thorax to be the two regions with appendages and the
abdomen to be the region without
appendages. Another interpretation is that the region of fused
segments is part of the abdomen.
Carapace
Note that a carapace is present but there is no
cephalothorax. No thoracic segment is fused with the head so there
is no cephalothorax. Carapace and cephalothorax are not the same and
should not be confused, although they often are.
The crustacean carapace is a posterior fold of the body
wall of the segment of the second maxilla, which is the posterior
edge of the head. It overhangs the body, to greater or lesser
extent, and may be attached to it. In Notostraca, the carapace
covers all of the thorax but is not attached to it at any point.
Head
Look at the dorsal surface again. The head bears a pair
of dorsal compound eyes (Fig 1, 19-13) that lie
close to each other near the midline. The compound eyes are sessile,
not stalked as are those of anostracans. In addition, there is a
naupliar eye on the anterior midline. The compound
eyes are on the dorsal surface of the head but the naupliar eye is
deep within the head. All the eyes are easily seen through the
integument of the head.
A distinct transverse groove, the mandibular
groove, marks the division between the anterior three head
segments and the posterior two (Fig 1). A second transverse groove,
the cervical groove, just posterior to the first,
marks the division between the head and thorax.
Head Appendages
Look at the ventral surface of the head. A lenslike
window on the ventral midline of the head admits
light to the ventrally aimed naupliar eye.
The first antennae are small, short,
slender filaments on the ventral surface of the head, at about the
level of the eyes. The second antennae are similar and located
lateral to the first. They are vestigial and inconspicuous. They are
absent in some species but are present in Triops longicaudatus.
The large, well-developed mandibles
oppose each other across the ventral midline. Their opposing median
surfaces bear strong brownish-yellow teeth. In living,
unanesthetized specimens you can watch the teeth move apart then
close together as the animal periodically opens and closes the
mandibles. Of the usual crustacean head appendages, only the
mandibles are well developed.
A transparent, unpaired, median labrum
arises from the body wall between the bases of the antennae and
extends posteriorly to cover the mouth and ventral ends of the
mandibles.
The first and second maxillae lie posterior to the
mandibles. They are small but bear distinct setae. The second
maxillae are larger than the first. The nephridiopores are located
on the second maxillae. (The second maxillae are absent in some
species.)
Trunk
In this exercise the trunk is considered to consist of a
thorax of appendage-bearing segments and abdomen of segments without
appendages. The anterior thorax consists of 11 segments and each
bears a pair of appendages, called thoracopods. The
segments of the posterior thorax are incompletely separated to form
rings. Each ring may consist of as many as six
fused segments and consequently may bear up to six pairs of
appendages. There may be up to 70 pairs of appendages on the entire
thorax. The genital segments are located between the two regions of
the thorax.
The posterior few rings of the trunk are the abdomen do
not bear appendages. The telson is the posterior
end of the trunk. It bears a caudal furca
consisting of two long, multiarticulate, whiplike rami (Fig 1,
19-13). The anus lies on the telson between the bases of the two
rami.
Trunk Appendages
Most of the thoracic appendages, or thoracopods,
resemble each other but the first 11 pairs are best developed. There
is a slight tendency to regional specialization and the first
thoracopod is unlike the remaining pairs. It has a sensory function,
replacing the reduced antennae in that role, whereas the remaining
anterior thoracic appendages (2-10) are the major locomotory,
feeding, and respiratory limbs.
The 11th appendages of females form brood pouches. The
many appendages posterior to the 11th move the spent feeding and
respiratory current away from the body and are also respiratory.
Most of the thoracopods are flat, leaflike
phyllopods derived from and resembling the ancestral
biramous crustacean appendage. The first thoracopod, however, is not
a phyllopod. As is true of anostracans, it is difficult to draw
exact homologies between the parts of the notostracan limb and that
of the ancestral limb. The names used here reflect possible
homologies but these are by no means certain and are questioned by
some crustacean specialists.
Begin with the second thoracopod skipping the unusual,
antenniform first thoracopod for the time being. Examine this
appendage while it still on the animal with high power of the
dissecting microscope. (If instructed to do so, remove this
appendage and make a wetmount of it for examination with the
compound microscope.)
Thoracopod 2
The central part of the appendage is the
protopod (Fig 2) whose proximal end is attached to the
body. On the lateral surface of the protopod are two exites. (Any
process from the lateral border of a crustacean limb is an exite and
any process from the medial border is an endite.) The proximal
process is the gill. It is teardrop-shaped and does not have
setae. The much larger, setose, distal exite is the exopod.
On the medial edge of the protopod there are several
endites. The distal endite is the endopod. It is
stiff, sharp and blade-shaped. The remaining endites
resemble the endopod but are smaller. The proximal endite is strong
and armed with spines on its medial margin. It is a
gnathobase. The two (right and left) gnathobases of each
pair of appendages are close to each other and face each other
across the midline. The remaining endites are farther from the
midline. The two rows of gnathobases form the right and left sides
of the conspicuous midventral food groove.
Figure 2. The second thoracopod (1 st phyllopod)
of Triops longicaudatus. Notostraca3L.gif
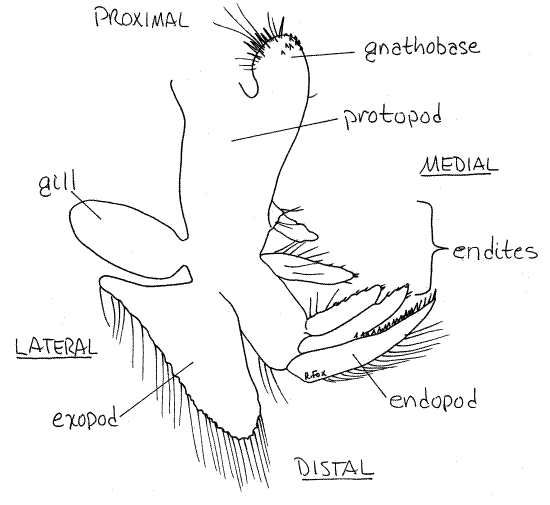
Thoracopod 1
The first thoracopod is modified to function as a
sensory structure. It has the same parts as other thoracopods but
they differ in morphology and function. Its protopod
is narrow. A gill and exopod are
present and resemble those of the phyllopods. The endopod
is reduced to a small, almost seta-less, distal process. The four
endites are long, multiarticulate flagella that
look and function like antennae (i.e. antenniform). The distal one
is longest and the proximal one is quite short. The gnathobase is
like those of the other trunk appendages. As the animal moves over
the substratum the antenniform flagella come in contact with it and
with potential prey. When such an object is detected by these
flagella the shrimp leaps onto it and covers it with the carapace.
The 11th pair of trunk appendages form brood pouches in
females. The protopod, gill, and exopods contribute to the
pouch. The protopod forms a cup for which the exopod is the
cover. These limbs are not modified in the male.
Feeding
The feeding method of notostracans is similar to that
proposed for the ancestral crustacean. The anterior phyllopods
(2-10) stir sediments and swirl muddy water and particles up into
the wide, midventral food groove. Motion of the gnathobases moves
food anteriorly in the food groove. The motion of the spiny
gnathobases can be seen in living specimens viewed from the ventral
surface.
The large flat exopods are primarily responsible for
stirring and lifting sediments. Fine silt particles and water escape
laterally but coarse particles, including food, remain in the
ventral food groove. Here they are torn into small pieces by the
sharp bladelike endopods and moved anteriorly to the mandibles and
mouth by the gnathobases. The mouth faces posteriorly to receive
food arriving in the food groove.
Figure 3. The first thoracopod of Triops longicaudatus. Notostraca2L.gif
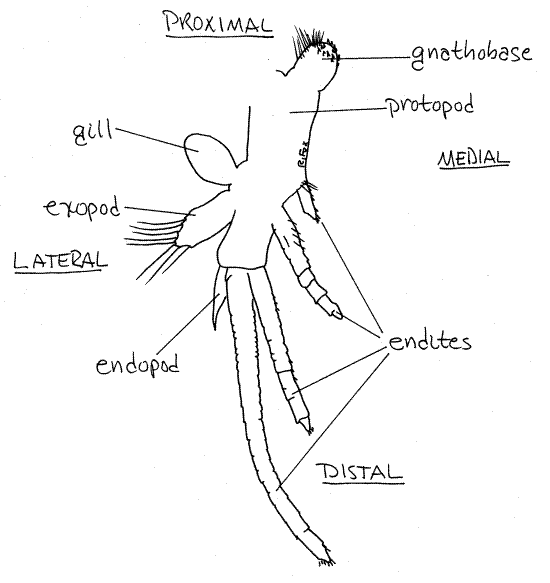
Particulate food includes small insect larvae,
oligochaete worms, and tadpoles. Notostracans may also engage in
suspension feeding while swimming. For this they use the setae of
the endites.
>1b. Feeding is easily observed in
living notostracans. To observe predation place a tadpole shrimp in
a small dish with some brine shrimp smaller than the tadpole. Small
oligochaetes such as Tubifex can also be used. Observe the
shrimp with the dissecting microscope. If you are patient you should
eventually see the tadpole shrimp discover a prey animal and leap
upon it. Continue watching as the powerful mandibles tear the prey
into small pieces which are then swallowed. <
>1c. Suspension feeding is also an
important feeding mode and can be demonstrated by placing a little
yeast/Congo red suspension in a dish with a tadpole
shrimp. Instructions for preparation of the stained yeast will be
found in the Supplies chapter.
Watch the shrimp continuously if you wish or set it
aside and return to it in about 30 minutes. The anterior end of the
gut (stomach) will quickly turn bright red as stained yeast cells
accumulate there. Soon the entire gut will be red. Pigment will
eventually appear in the branched digestive ceca in the head. This
is the best way to see digestive system. Return to this preparation
when you study the gut. <
Internal Anatomy
Most internal features are difficult to see from the
outside. The heart is a long, dorsal tube in the anterior 11 trunk
segments. It has a pair of ostia in each of these
segments. Hemoglobin is sometimes present in the blood and the
animal may be pink as a result.
The excretory/osmoregulatory organs are the paired
maxillary glands (= saccate nephridia) in the
segment of the second maxilla (Fig 1). The long looped ducts of
these glands can be seen in the carapace (Fig 1, 19-13). The role of
the maxillary glands is primarily osmoregulatory. Nitrogen, in the
form of ammonia, is lost by diffusion across the gill surfaces.
The mouth opens between the two mandibles on the ventral
surface of the head. A short, vertical esophagus connects it with
the stomach in the head. Two digestive ceca have branches extending
into the carapace. The intestine extends posteriorly through the
trunk to join a short rectum which opens at the anus. The intestine
is easily seen.
>1d. If you have living specimens and
have not already done so, place some yeast/Congo red suspension in
the dish with a shrimp. After 15-30 minutes examine the animal with
the dissecting microscope. The gut, now filled with red pigment, is
easily seen. If necessary, and if there are plenty of specimens, add
a drop of chloroform to the water in the dish to stop the motion of
the animal. This may kill the specimen so don't do it if living
animals are in short supply. <
The paired gonads extend almost the entire length of the
trunk on either side of the gut. They open via gonopores on the 11th
pair of thoracopods.
Parthenogenesis is common and males may be rare. Females
produce thin-shelled summer eggs or thick-shelled resting eggs which
survive freezing and desiccation. Eggs hatch as nauplii or
metanauplii.
References
Kaestner, A. 1970.
Invertebrate zoology, Crustacea, vol III. Wiley Interscience, New
York. 523pp.
Lankester ER
. 1881. Observations and
reflections on the appendages and on the nervous system of Apus
cancriformis. Quart. J. Micros. Sci. 21:343-
Linder F. Contributions
to the morphology and taxonomy of the Branchjiopoda Notostraca, with
special reference to the North American species. Proc. US Nat. Mus.
102(3291):1-69, pls 1-7.
Longhurst
AR . 1955. A
review of the Notostraca. Bull. Brit. Mus. Nat. Hist. Zool
3(1):1-57.
Martin JW. 1992.
Branchiopoda, pp25-224 in Harrison FW, Humes AG (eds.) Microscopical
anatomy of invertebrates, vol 9 Crustacea. Wiley, New York. 652pp.
Pennak RW. 1978. Fresh-water
Invertebrates of the United States 2 nd ed. Wiley, New
York. 803pp.
Ruppert EE, Fox RS, Barnes
RB. 2004.
Invertebrate Zoology, A functional evolutionary approach, 7 th
ed. Brooks Cole Thomson, Belmont CA. 963 pp.
Tasch P. 1969. Branchiopoda,
in R. C. Moore (ed) Treatise on Invertebrate Paleontology, pt R:
Arthropoda 4(1). Geological Soc. America, Boulder.
Supplies
Dissecting microscope
Compound microscope
Living or preserved Triops
8-cm culture dish
Chloroform
Yeast/Congo red suspension
Lab Prep
The teaching staff should hatch eggs ("living fossils")
purchased from Ward's Natural Science Co. and provide the class with
living (or perhaps preserved if specimens have been saved from
classes in previous years) adults and juvenile stages to
study. Usually only a few tadpole shrimp hatch from each vial of
detritus and several vials will be required to provide an entire
class with living specimens. Fairy shrimp will also be present. The
soil is collected from the bottom of temporary ponds in Utah.
It may be desirable to preserve the specimens used each
year for use in subsequent years in the event that insufficient
living animals are available. Some instructors, especially those
with large classes, may want to use preserved material for study of
anatomy but provide a few living specimens for behavioral
observations. Material to be preserved should first be fixed in 5%
formalin overnight, washed thoroughly in freshwater, and then stored
in 80% ethanol or 40% isopropanol.
To hatch the eggs empty the contents of the vial as
received from Ward's into a large fingerbowl of chlorine-free
freshwater. The eggs hatch quickly (24 hours) and grow
rapidly. Tadpole shrimps are carnivorous and will eat any other
small soft-bodied animals in the dish, including each other and the
fairy shrimp that are also present. Newly hatched nauplii begin to
disappear almost as soon as they hatch when they fall prey to their
slightly larger siblings. To maximize the production of shrimp, each
nauplius should be removed to its own small culture dish as soon as
it appears. The fairy shrimp developing in the culture can be
studied using the Artemia exercise in this collection
.
Tadpole shrimp can be fed a yeast suspension and/or
Artemia larvae and juveniles. Avoid use of formalin- or
soap-contaminated glassware or instruments when rearing larvae.