Invertebrate Anatomy OnLine
Tibicen spp ©
Cicadas
30jun2006
Copyright 2001 by
Richard Fox
Lander University
Preface
This is one of many exercises available from Invertebrate Anatomy OnLine , an Internet laboratory manual for courses in Invertebrate Zoology. Additional exercises can be accessed by clicking on the links on the left. A glossary and chapters on supplies and laboratory techniques are also available. Terminology and phylogeny used in these exercises correspond to usage in the Invertebrate Zoology textbook by Ruppert, Fox, and Barnes (2004). Hyphenated figure callouts refer to figures in the textbook. Callouts that are not hyphenated refer to figures embedded in the exercise. The glossary includes terms from this textbook as well as the laboratory exercises.
Systematics
Arthropoda P, Mandibulata, Tracheata, Hexapoda SC, Insecta C, Dicondylia, Pterygota, Metapterygota, Neoptera, Eumetabola, Paraneoptera, Hemiptera O, Homoptera sO, Auchenorrhyncha iO, Cicadoidea SF, Cicadidae F, Tibicen spp. (Fig 16-15, 20-14, 20-15, 21-23)
Arthropoda P
Arthropoda, by far the largest and most diverse animal taxon, includes chelicerates, insects, myriapods, and crustaceans as well as many extinct taxa. The body is segmented and primitively bears a pair of jointed appendages on each segment. The epidermis secretes a complex cuticular exoskeleton which must be molted to permit increase in size. Extant arthropods exhibit regional specialization in the structure and function of segments and appendages. The body is typically divided into a head and trunk, of which the trunk is often itself divided into thorax and abdomen.
The gut consists of foregut, midgut, and hindgut and extends the length of the body from anterior mouth to posterior anus. Foregut and hindgut are epidermal invaginations, being derived from the embryonic stomodeum and proctodeum respectively, and are lined by cuticle, as are all epidermal surfaces. The midgut is endodermal and is responsible for most enzyme secretion, hydrolysis, and absorption.
The coelom is reduced to small spaces associated with the gonads and kidney. The functional body cavity is a spacious hemocoel divided by a horizontal diaphragm into a dorsal pericardial sinus and a much larger perivisceral sinus. Sometimes there is a small ventral perineural sinus surrounding the ventral nerve cord.
The hemal system includes a dorsal, contractile, tubular, ostiate heart that pumps blood to and from the hemocoel. Excretory organs vary with taxon and include Malpighian tubules, saccate nephridia, and nephrocytes. Respiratory organs also vary with taxon and include many types of gills, book lungs, and tracheae.
The nervous system consists of a dorsal, anterior brain of two or three pairs of ganglia, circumenteric connectives, and a paired ventral nerve cord with segmental ganglia and segmental peripheral nerves. Various degrees of condensation and cephalization are found in different taxa.
Development is derived with centrolecithal eggs and superficial cleavage. There is frequently a larva although development is direct in many. Juveniles pass through a series of instars separated by molts until reaching the adult size and reproductive condition. At this time molting and growth may cease or continue, depending on taxon.
Mandibulata
Mandibulata includes arthropods in which the third head segment bears a pair of mandibles. As currently conceived this taxon includes myriapods, hexapods, and crustaceans. Appendages may be uni- or biramous and habitats include marine, freshwater, terrestrial, and aerial.
Tracheata
Myriapods and hexapods share tracheae and a single pair of antennae and are sister taxa in Tracheata. Crustaceans, which have gills and lack tracheae, are excluded and form the sister group.
Hexapoda
The body is divided into three tagmata; head, thorax, and abdomen. Appendages are uniramous and a single pair of antennae is present. Three pairs of legs and two pairs of wings are found on the thorax of most adults. Hexapod legs are uniramous although there is increasing evidence that they evolved from multiramous appendages of their ancestors. Gas exchange is accomplished by trachea. Excretory organs are Malpighian tubules and the end product of nitrogen metabolism is uric acid. There is relatively little cephalization of the nervous system. Insects are gonochoric with copulation and internal fertilization.
Insecta C
Most hexapods are insects. A few hexapod taxa (orders) lack wings and have primitive mouthparts recessed into the head and belong to Entognatha, the sister taxon of Insecta. Insects have ectognath mouthparts and the adults (imagoes) of most taxa have wings.
Pterygota
The winged insects. These insects are derived from a winged common ancestor. Adults of most taxa have wings although they have been lost in some.
Eumetabola
Juveniles have no ocelli and there are six or fewer Malpighian tubules.
Paraneoptera
The hemipteroid, or bug-like, insects. Most have sucking mouthparts. Development is paurometabolous with nymphs that resemble the adults except for size and lack of sexual maturity and wings. Nymphs have wingpads.
Hemiptera O
Sucking mouthparts and liquid, either animal or plant, diet. Mouthparts a beak consisting of a piercing stylet supported by the labium. The stylet is composed of four needle like processes, two of them maxillary and two mandibular. Imagoes have two pairs of wings, of which the hindwings are membranous. Members of the suborder Homoptera have membranous fore- and hindwings whereas the forewings of Heteroptera are partly leathery and partly membranous hemielytra. This is the largest order of non-holometabolous insects with about 80,000 species and are the only insects correctly referred to as bugs.
Homoptera sO
This ecologically and economically important suborder includes cicadas, leafhoppers, treehoppers, planthoppers, whiteflies, aphids, adelgids, scale insects, and many others. The forewings and hindwings are both entirely membranous and there are no hemielytra. The beak attaches to the posterior head. Many entomologists consider Homoptera to be an order distinct from Hemiptera (Heteroptera) and others consider it to be paraphyletic. All are herbivorous feeding on plant juices, with major taxa tending to specialize in either phloem or xylem which they access using the sucking beak.
Introduction
Cicadas are common and familiar insects, often called “locusts” (which they are not) or harvest flies. The din created by singing males is a familiar feature of summer in most of temperate North America. At least one species, and often several, is likely to be available in summer months and, in the eastern United States, sometimes in the spring. About 100 species are known from the United States. Although large, cicadas are difficult to dissect and are not ideal subjects for the study of general insect anatomy. The gut and tracheal systems are highly derived and the nervous system moderately so, being more condensed than that of primitive insects. This exercise is limited to external anatomy of adults and nymphs. Color photographs, keys, and discussions of biology and anatomy are available in Myers (1929), Borrer et al. (1989) Cooley, et al. (2000), and Hickernell (1920).
General Biology
Although common and large (body length 25-50 mm, excluding wings), cicadas are only occasionally seen because the juveniles live underground and adults spend most of their time in the tops of trees. Occasionally an adult will be found on or near the ground and more commonly the empty exuvium of a final instar nymph will be found clinging to the bark of a bush or tree. Adults and nymphs do not resemble each other and most people do not make the association. One is much more likely to hear, than see, cicadas but during an emergence adult periodical cicadas may be so common as to be sighted frequently.
Types of Cicadas
The 1500 known species of cicadas belong to Cicadidae and are found in most warm parts of the world. In the United States about 100 species are known, with the most common belonging to two genera. The widely distributed dog-day cicadas (annual cicadas, or harvest flies) in the genus Tibicen are characteristic of late summer (July and August). The periodical cicadas (Magicicada) of spring (May and June) are endemic to the eastern United States as far west as Oklahoma and Texas. There are also other North American genera, includingDiceroprocta, Neocicada, and Okanagana. Adult dog-day cicadas appear every year in late summer and tend to have black and green bodies and wing veins. Adult periodical cicadas appear only once every 13 or 17 years, have red or orange eyes, reddish or black body, red wing veins, and tend to be smaller than dog-day cicadas. This exercise is based on examinations of Tibicen but applies as well to Magicicada.
Most cicadas have transparent wings with pigmented veins. Body colors are due to pigments in the cuticle or to subcutaneous tissues showing through transparent areas of the cuticle. Many species are pruinose, that is, covered by a waxy white powder. Setae appear on many parts of the body, sometimes in dense patches. Pubescent patches of setae may be visible as areas of contrasting color. Setae have been omitted from the drawings in this exercise to improve clarity.
Life Cycles and Periodicity
The cicada life cycle consists of egg, several nymphal instars, and the imago. There is no pupa. The female uses a blade-like ovipositor to cut a slit in the tip of woody branches into which eggs are deposited. After hatching the first instar nymphs fall to the ground and burrow into it. Many years are spent feeding underground as nymphs in cells or burrows in the soil before the final nymphal instar burrows out of the soil, crawls up a tree or shrub, splits its exoskeleton along the dorsal midline, and emerges as an imago. The distinctive nymphal exuvium is left behind hanging onto the bark and is a familiar sight to most people.
Dog-day and periodical cicadas differ importantly in the length of the nymphal phase of the life cycle and in synchronization of emergences. Although hemipterans are typically paurometabolous, cicadas resemble hemimetabolous insects in that their nymphs do not closely resemble the adults.
The nymphal phase of periodical cicadas (Magicicada) is either 13 or 17 years. Emergences are synchronized so that in any given geographic region experiences an emergence (of a given species) only every 13 or 17 years. In years between emergences there are no Magicicada adults (or, if there are, they belong to a different species) but during emergences they can be spectacularly abundant, presumably overwhelming potential predators so that large numbers survive to reproduce. Six species of periodical cicadas are known. Three southern species have 13-year life cycles whereas three northern species have 17-year cycles. The southern and northern species occur in sibling species pairs with similar morphological, behavioral, and acoustic characteristics typical of each pair. Furthermore, each species is composed of several broods, probably 13 broods of 17-year cicadas and 5 broods of 13-year cicadas. Each brood emerges in a different year and inhabits a different range. Any given geographic region may support more than one species and more than one brood. Thirteen and 17 year species can coexist as can different broods of each. Thus there can be emergences of periodical cicadas at intervals other than 13 or 17 years but the emergences would belong to different broods or species. Adult life span is 2-6 weeks.
In contrast, dog-day cicadas (Tibicen) have a nymphal life of uncertain length but probably about 2-7 years. These cicadas emerge, mate, and oviposit each year and their emergences are not synchronized. Adult dog-day cicadas are present every summer.
Songs
The male “vocalizations” are species-specific and experienced observers can identify the species from the song alone. Cicada songs can be painfully loud, perhaps even deafening, and are probably the loudest sounds made by insects. Sound pressure levels as high as 108 decibels have been recorded for individual cicadas measured 50 cm from the insect (Petti, 1997). (A lawnmower is about 100 dB and a chainsaw is 110 dB). Volume is positively correlated with body weight. A calling song is produced to attract conspecific females, and in some species males also, into mating aggregations. Under some circumstances it may repel males. The alarm call, which differs from the calling song, may inhibit predation or decrease predatory efficiency.
Sound is produced by tymbals on the dorsal anterior abdomen of the male. Sound reception is the function of eardrums, or tympana, which are thin sheets of cuticle located ventrally on the anterior abdomen, adjacent to the tymbals. The tympana are covered by a posteriorly projecting thoracic flap, or operculum (Fig. 6). Tympana are present in both sexes although they are much larger in males. Tymbals are present only in males.
Food
Adults feed using their beak to tap into the xylem of the aerial portions of woody plants. The water from the xylem is probably as important or more important than the food. The subterranean larvae feed from the xylem of the roots of trees. Although both nymphs and imagoes feed from xylem the only important damage to plants results from oviposition in young twigs, which may cause extensive dieback, particularly in years of periodical cicada emergences.
As xylem feeders, cicadas rely on an exceedingly dilute food resource as the function of xylem is to transport water and mineral nutrients, not food. Perhaps leakage of carbohydrate from intact or damaged phloem into nearby xylem vessels provides cicadas with sufficient organic compounds. This apparent disadvantage of xylem feeding may be countered by access to a potentially abundant supply of water from the xylem. Such a resource could be valuable on hot summer afternoons and at least one species is known to use the water for thermoregulation by evaporative cooling. Diceroprocta apache in the Sonoran Desert, survives in temperatures up to 48 ° C using water from the xylem of mesquite. Mesquite is a desert shrub or small tree whose roots extend deep into the soil to maintain contact with the groundwater (Toolson & Hadley, 1987).
Specimens
This exercise is written specifically for living or freshly sacrificed adult cicadas in the genus Tibicen. The exercise is easily adapted to other genera, such as Magicicada or Diceroprocta. Preserved material can be used if necessary but when available, fresh, unpreserved material is preferable. Dried specimens can be used for the study of external anatomy/. Color and texture descriptions may not be applicable to preserved material and, of course, there is no opportunity for behavioral or physiological observations. The study should be conducted with a dissecting microscope.
Cicada Anatomy
Adults
Study the external anatomy of an adult cicada of either sex. Living specimens should be anesthetized using chloroform or killed using ethyl acetate. A small cotton ball should be slightly (!) moistened with the appropriate fluid and placed in a container with the specimen. Dried specimens can also be used. The study should be conducted with a dissecting microscope.
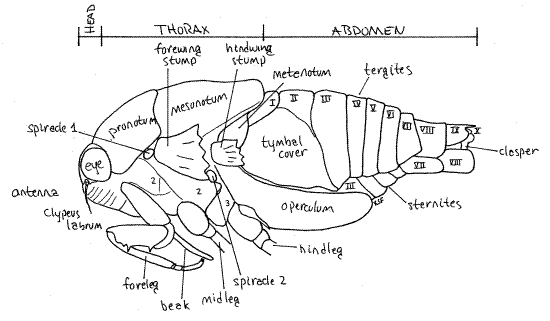
The body consists of three tagmata, head, thorax, and abdomen (Fig. 1, 16-2*).
Figure 1. View of the left side of an adult cicada in the genusTibicen. The wings, midlegs, and hindlegs have been removed for clarity. Homopt11L.gif
Head
The head is the smallest of the three tagmata (Fig 21-1A,B). It consists of a hard, sclerotized head capsule dominated by two bulging lateral compound eyes, a bulging median clypeus, and a long, slender, median beak.
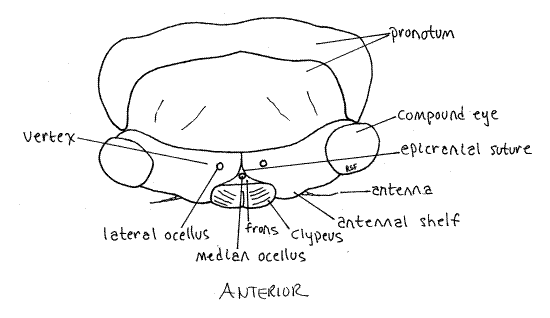
The median dorsal region of the head capsule the vertex from which the large compound eyes protrude laterally on thick eyestalks (Fig. 2). The Y-shaped epicranial suture divides the top of the head capsule into right and left vertices and a tiny median region, the frons (Fig. 2). The epicranial suture consist of three smaller sutures. The stem, which divides the vertex into two halves, is the coronal suture. It is the stem of the “Y”. The two arms of the epicranial suture are the frontal sutures which divide the frons from the vertex. The frons lies on the anterior border of the vertex between the two arms of the "Y". Lateral to the frons, the vertices extend anteriorly to form small antennal shelves overhanging the bases of the antennae.
Figure 2. Dorsal view of the head of an adult cicada, Tibicen. Homopt12L.gif
The antennae are short, very slender, and consist of 7-9 articles, depending on species (Fig. 1). The two proximal articles, partially hidden by the shelf, form the antennal peduncle and the remaining articles are the flagellum.
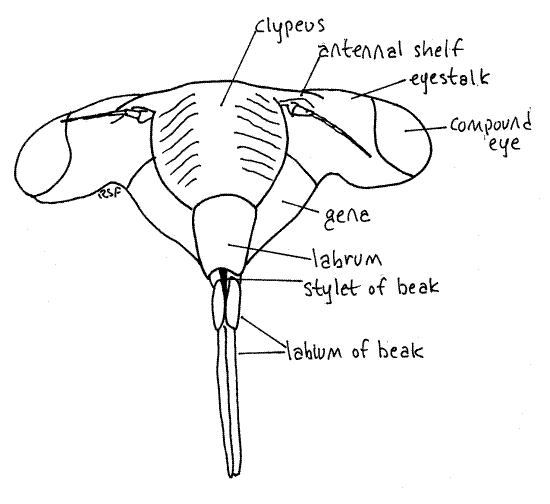
Medially, the anterior border of the frons articulates with the clypeus, which is another region of the head capsule (Fig. 2). In dorsal view the clypeus appears to be a relatively small semicircular sclerite occupying the center of the margin of the top of the head. It is actually much larger and occupies much of the anterior face of the capsule (Fig. 3).
Figure 3. En face view of the head of an adult cicada, Tibicen. Homopt13L.gif
The gem-like cuticular corneas of one median ocellus and two lateral ocelli are visible on the head capsule between the two compound eyes (Fig. A). The lateral ocelli are located on the vertex but the median eye is on the frons.
Posteriorly the vertex curves inward as the occiput of the capsule. Pull the head slightly forward, away from the thorax to see the narrow curved occiput. This will also reveal the soft, unsclerotized cervix, or neck, connecting head with thorax. The large opening in the posterior head capsule, between head and thorax and covered by the cervix, is the foramen magnum. All tissues passing between head and thorax pass through the foramen. You cannot actually see the opening because it is covered by the cuticle of the cervix.
Hold the cicada so that you can view the anterior head. This is the en face view, or view of the face (Fig. 3). The head in this view resembles a trilobite. The clypeus forms a prominent, striated bulge on the midline with the eyestalks and compound eyes extending laterally. The labrum is a smaller, non-striated bulge attached to the ventral border of the clypeus. There is disagreement in the literature about the correct names for these sclerites and the interpretation followed is that of Comstock (1920). According to Borrer, et al (1989) the structure referred to above as the labrum is part of a two-lobed clypeus.
The antennal shelf and antenna lie on each side of the clypeus (Fig 3). The cheeks, or genae extend ventrally beside the clypeus. This region of the gena is sometimes known as the lorum.
The mouthparts of Hemiptera, both Heteroptera and Homoptera, are adapted for piercing and sucking. They include parts of the mandibles, maxillae, and labium which collectively form a prominent beak, or rostrum, extending from the ventral extremity of the labrum (Fig. 3, 21-4). The beak consists of the modified labium, which forms a support for a slender delicate stylet made of the mandibles and maxillae. Most of what you see on your specimen is the labium. It is a sturdy, 3-jointed structure with a distinct, deep groove on its anterior surface (Fig. 4). The dark sclerotized stylet can be seen emerging from the tip of the labrum and disappearing into the groove. In use, the labium folds at its joints and leaves the stylet exposed to penetrate the prey. The labium does not penetrate.
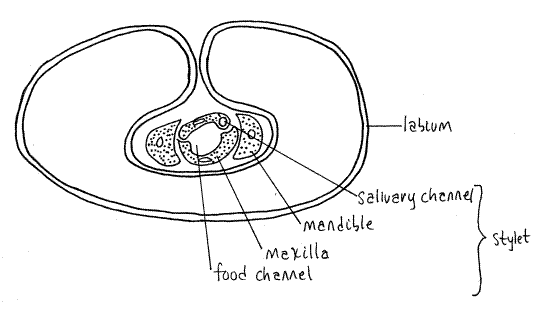
The stylet consists of a long slender process from each mandible and each maxilla, for a total of four processes. The two maxillae together form a central tube with two channels, one inbound for food and leading to the mouth, the other outbound for saliva and leading from the salivarium and salivary ducts (Fig. 4). The mandibles form a tube around the maxillae. Maxillae and mandibles are equipped with protractor and retractor muscles.
Figure 4. Cross section of the third segment of a cicada beak. Redrawn from Comstock (1920). Homopt14L.gif
A heavily muscularized sucking pharynx under the bulging clypeus is the pump for bringing liquid food into the gut. The striations characteristic of the cicada clypeus are the external manifestation of ridges for the insertion of the dilator muscles of the pharyngeal pump.
Thorax
The thorax consists of anterior prothorax, middle mesothorax, and posterior metathorax (Fig 1) The prothorax and mesothorax are large but the metathorax is much smaller. Of the three, the mesothorax, containing the flight muscles for the forewings, is the largest. The metathorax is not visible dorsally. Each of the three thoracic segments has a pair of legs and the mesothorax and metathorax each have a pair of wings.
The prothorax is enclosed by a large dorsal pronotum and a much smaller ventral prosternum, with the pleuron between them laterally. These sclerites are fused to form a single cuticular ring around the segment. The pronotum, which has a wide, girdle-like posterior flange that overlaps the mesonotum, forms a shield over the dorsal and lateral aspects of the segment (Fig. 1, 2). The prosternum lies on the ventral midline and extends laterally to the pleural region. It is narrowest on the midline where it forms a groove in which the beak rests. The foreleg articulates with the unsclerotized articulating membrane of the pleuron.
The large mesothorax is covered dorsally by the sclerotized mesonotum (Fig. 1, 5). The chief ventral sclerite of this segment is a large mesosternum anterior to the legs and posterior to the prosternum but there are also several smaller sternal and pleural plates in the vicinity of the midlegs and forewings respectively.
The metathorax is the smallest of the thoracic segments. Its metanotum is inconspicuous, being overlaid by a posterior flange of the mesonotum, but is visible laterally beside the bases of the hindwings and hidden by the hindwings (Fig. 5). The metasternum is visible ventrally as a narrow transverse sclerite anterior to the coxae of the hindlegs. In Tibicen it bears a median blisterlike tubercle. A pointed, bladelike sclerite, the metacanthus, extends away from the body beside the base of the metacoxa (Fig. 1, 6). A pair of platelike opercula extend posteriorly from the ventral metathorax to cover an auditory chamber containing the sound-receiving tympana (tympanic membranes) on the anterior ventral abdomen (Fig. 6). Opercula and tympana are present in both sexes but the opercula of males are very large whereas those of females are much smaller.
Figure 5. Dorsal view of the abdomen of the cicada, Tibicen. Homopt15L.gif
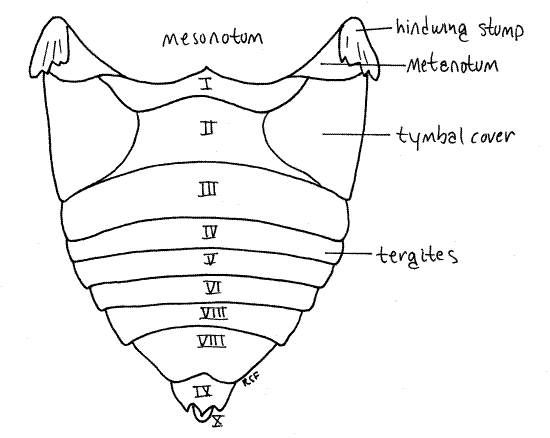
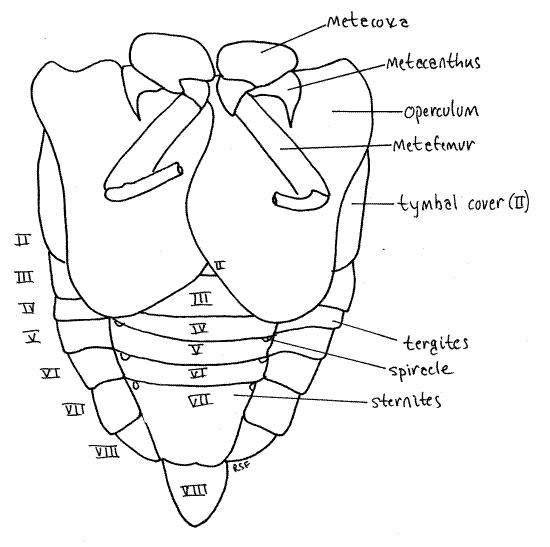
Figure 6. Ventral view of the abdomen of the cicada, Tibicen. The hindlegs have been truncated for clarity. Homopt16L.gif
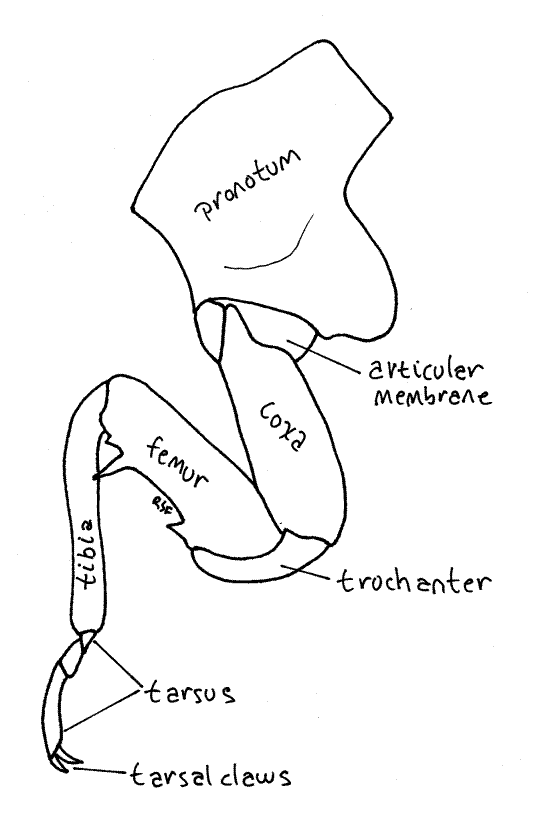
Each thoracic segment bears a pair of legs known, in order, as the forelegs, midlegs, and hindlegs. The foreleg (Fig. 7, 21-1E) begins with an elongate coxa which articulates with the pleuron. A smaller trochanter connects the coxa with the femur. The femur is enlarged and forms a prehensile organ in conjunction with the tibia. One border of the femur, the palm, is toothed and faces the tibia, which closes against it. The long slender tibia is the movable finger of the prehensile organ. Flexor muscles in the femur pull it against the femur. The tarsus consists of three articles and terminates in a pair of tarsal claws.
Figure 7. Left foreleg of an adult cicada, Tibicen. Homopt17L.gif
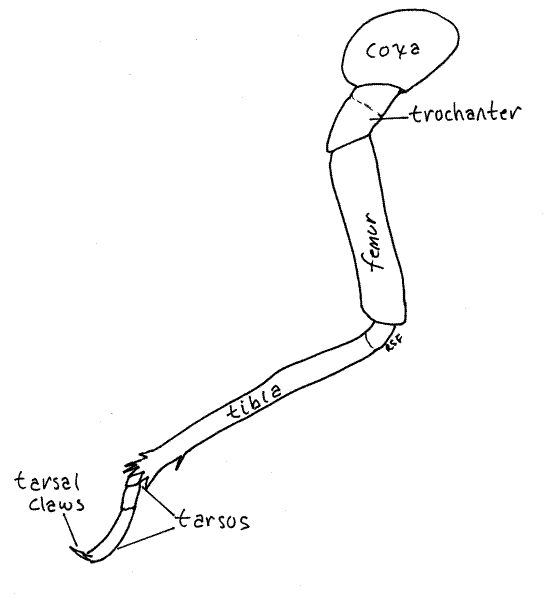
The midlegs and hindlegs differ from the forelegs in many respects but are similar to each other (Fig. 8). The hindlegs are the larger. The coxa is short and wide, unlike that of the forelegs, and the femur and tibia are not part of a prehensile hand.
Forewings and hindwings are borne on the mesothorax and metathorax respectively (Fig. 1). The large, veined, transparent wings are held at angles, roof-like, over the body. Each wing arises from a cluster of small sclerites in the pleural region on the sides of its segment. The forewings are the larger of the two pairs. The wings are large, membranous, and transparent with dark structurally important veins. The heavy leading edge of the wing is the combined costal and subcostal veins.
In flight the forewing and hind wing are locked together by a catch mechanism consisting of a fold in the proximal posterior edge of the forewing and a complementary fold in the proximal anterior edge of the hindwing. These two folds hook together so that the two wings on each side behave aerodynamically as a single airfoil.
Figure 8. Left hindleg of an adult cicada, Tibicen. Homopt18L.gif
Cicadas have 10 pairs of spiracles, 2 on the thorax and 8 on the abdomen. The thoracic spiracles are located laterally on the mesothorax and metathorax (Fig. 1) but both pairs can be difficult to locate. The mesothoracic spiracle (spiracle 1) is under the posterolateral corner of the pronotum, just anterior to the base of the forewing. It is wedged between the prothorax and mesothorax. The metathoracic spiracle (spiracle 2) is located on the anterior edge of the metathorax between the bases of the two wings. It is on the suture separating meta- and mesothorax.
Abdomen
The adult abdomen consists of 10 segments (Fig. 1, 5, 21-1F). A typical abdominal segment, such as 3-8, is enclosed by a dorsal tergite and ventral sternite. The tergite is a large arch covering dorsum, sides, and lateral aspect of the venter, whereas the sternite is much smaller, covering only the center of the venter (Fig. 6).
First study the abdomen in dorsal view (Fig. 5). Only a small median portion of the tergite of segment 1 is visible behind the posterior projection of the mesonotum and between the two tymbal covers. Tergite 2, however, is much larger. especially in males, in which it bears large, lateral, platelike tymbal covers (Fig. 1, 5). These extend anteriorly from the tergite and cover the sound producing tymbals, which will be discussed later. Tymbal covers are absent in Magicicada and Okanagana, and of course, are not present in females. They are greatly reduced in Neocicada. Tergites 3-7 are unremarkable and diminish in width posteriorly as the abdomen tapers. Tergite 8 is longer then 7 and tapers rapidly posteriorly. The tergite of segment 9 bears a median blunt or acute point, depending on species.
Examine the venter of your specimen (Fig. 6, 9). In males, much of the anterior abdomen is obscured by the opercula, which extend posteriorly from the metathorax to cover the tympanum (Fig. 6). In females the opercula are much smaller and cover very little of the abdomen (Fig. W). Each operculum covers an auditory chamber whose posterior wall is the tympanic membrane. These are thin cuticular eardrums used for sound reception. They will be dissected later.
Figure 9. Ventral view of an adult female cicada in the genus Tibicen. Homopt19L.gif
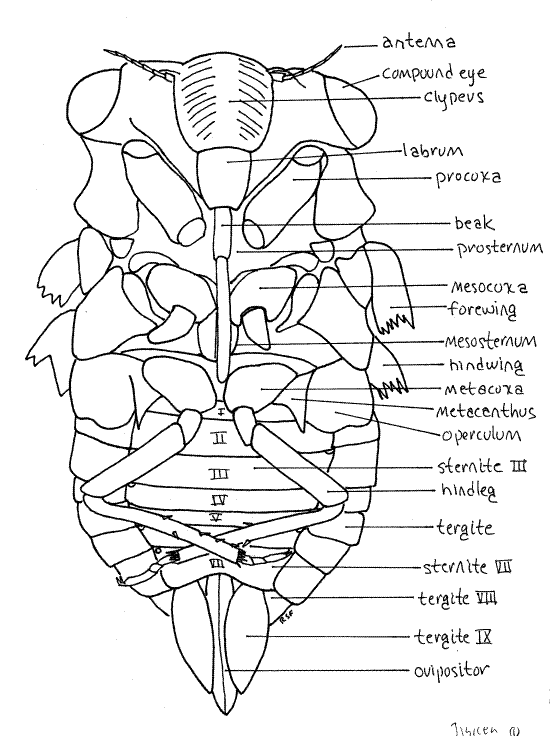
The sternites of segments 2-6 extend as bands across the venter of the abdomen. Sternite 7 is large and extends posteriorly to cover much of sternite 8.
Segment 8, 9, and 10 bear the external genitalia. Segment 8 is the pregenital segment, segment 9 the genital segment, and segment 10 the postgenital segment. Segments 7-10 differ in males and females and are discussed separately under "External Genitalia".
Abdominal spiracles are situated on the anterior lateral corners of the sternites of segments 1-8 (Fig. 6). They are small white ovals that may be difficult to see under the waxy white powder of pruinose specimens. Use a fine needle to scrape the powder away to reveal the spiracles.
External Genitalia
Female
Tergite 7 is a large arched sclerite (Fig. 10) and sternite 7 is a median, ventral, bilobed plate (Fig. 11).
Figure 10. Dorsal view of the posterior abdomen of a female cicada, Tibicen. Homopt20L.gif
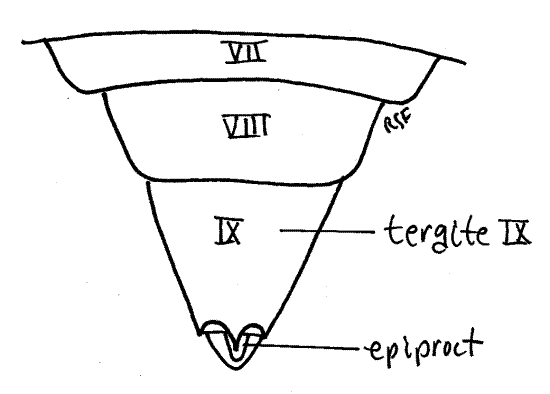
Figure 11. Ventral view of the posterior abdomen of a female cicada, Tibicen. Homopt21L.gif
The tergite 8 is a large dorsal arch that is scarcely visible ventrally. The posterior wall of segment 8 is invaginated to form the genital chamber into which the oviduct opens via the female gonopore.
Tergite 9 is a large, sclerotized, dorsal hood covering the genital chamber (Fig. 12). It almost completely encircles the segment and covers all of the dorsum and sides as well as most of the venter. A narrow longitudinal slit is left exposed along the ventral midline through which the ovipositor is visible.
Figure 12. Dorsal view of the posterior abdomen of a female cicada, Tibicen. Homopt22L.gif
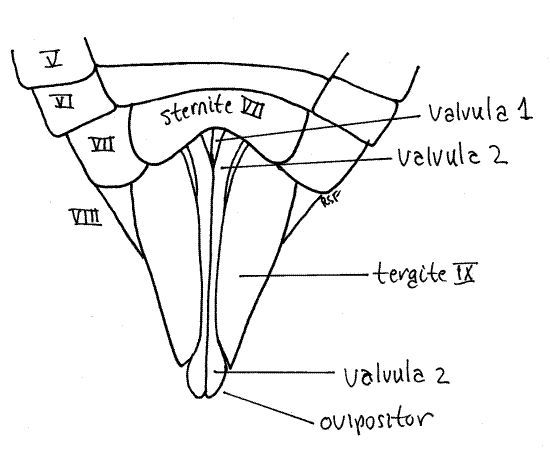
The ovipositor of Homoptera is large and well developed (that of Hemiptera is not) and resembles the ovipositor of other pterygotes. It is a posteriorly directed shaft consisting of two sets of interlocking sclerotized processes known as valvulae (Fig. 11, 21-11B). The first valvulae arise ventrally on segment 8 and the second on segment 9. In ventral view and at rest the second valvulae cover most of the length of the first valvulae. Most of the ovipositor is covered by tergite 9.
In cicadas the two second valvulae are fused to form a single piece. The two first valvulae and the single (fused) second valvula lie close together and are interlocked to form the tubular ovipositor, with the first valvulae ventral and the second valvula dorsal. Eggs pass through the lumen of this tube during oviposition into slits the female cuts in the tips of twigs. A third pair of valvulae, also arising on segment 9, form a sheath over the ovipositor but are not visible in ventral view.
Distally the first valvulae are armed with serrated blades used by the female to saw slits into the tips of the twigs in which eggs will be deposited (Fig. 13). Look at the distal tip of the ovipositor with about 20X magnification and use forceps and a minuten nadel to push the tips of the second valvulae apart. You can now see the tips of the first valvulae with their lateral serrations.
Figure 13. Posterior tip of the second valvulae of a female cicada in the genus Tibicen. Homopt23L.gif
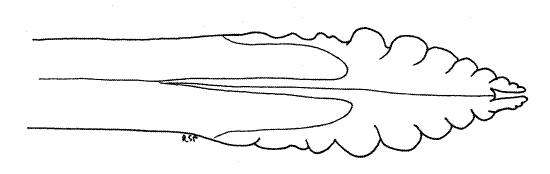
Segment 10 is small and projects posteriorly from the genital chamber and lies ventral to tergite 9 (Fig. 12). The vestigial segment 11, represented by little more than a single median epiproct and a pair of lateral paraprocts, is borne on the posterior 10 th segment (Fig. 10, 12).
Male
In males sternite 8 is a large scoop-shaped ventral plate (Fig. 14). Anteriorly it is covered by tergite 7 but posteriorly it extends under segment 9 where it protects the external genitalia. Sternite 8 underlies and supports segments 9-10 (Fig. 1). Tergite 8 is considerably longer than tergite 7 but otherwise unremarkable.
Figure 14. Lateral view of the posterior abdomen of a male cicada, Tibicen. Homopt24L.gif
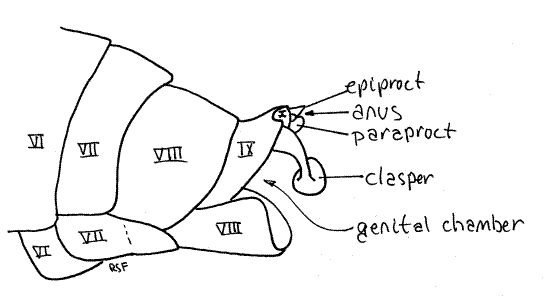
Tergite 9 is heavily sclerotized and bears a median, posterior, tooth whose size and shape varies with species (Fig. 15). A large posterior invagination, the genital chamber lies under tergite 9 and contains most of the male external genitalia, including a median, tubular, intromittent organ and the gonopore. Although known as "external" genitalia, they are not visible without dissection. The gonopore is on segment 9.
Figure 15. Dorsal view of the posterior abdomen of a male cicada, Tibicen. Homopt25L.gif
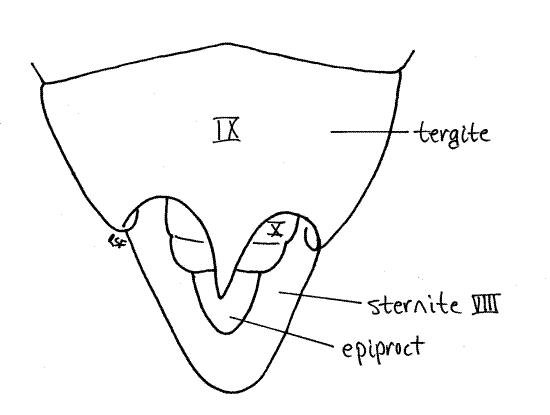
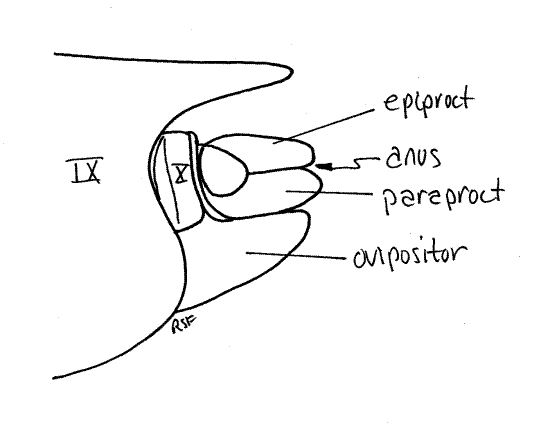
Segment 10, sometimes known as the anal segment, is much smaller than 9 and is nestled under tergite 9 (Fig. 14, 15). It can be seen protruding slightly posteriorly from beneath tergite 9. Its most conspicuous feature is a pair of heavily sclerotized copulatory claspers, which may be homologous to the cerci of other insects, and may be fused ventrally. The anal segment bears the terminal anus, which is covered dorsally by the median epiproct and laterally by two paraprocts (Fig. 14, 21-12B). These sclerites are all that remains of the vestigial 11 th abdominal segment.
Sound Equipment
Tymbal and Sound Production
Sound is produced by a pair of tymbals located dorsolaterally in tymbal chambers on the male first abdominal segment. In Tibicen and Diceroprocta the tymbals are covered by an anteriorly projecting flap of tergite 2 known as the tymbal cover but in Magicicada and Okanagana they are exposed. In Neocicada tymbal covers are present but small and inconspicuous.
Remove both wings from the left side of the body by cutting across the veins close to the body, leaving only a small stump. Use fine scissors to remove one of the tymbal covers from abdominal tergite 2 of a male. Make a transverse cut just a little anterior to the posterior border of tergite 2. Removing the cover will reveal the tymbal cavity between the first and second abdominal segments. The tymbal occupies the floor of this cavity. If your specimen belongs to Magicicada or Okanagana there will be no tymbal cover and the tymbal will be visible after the wings are removed.
Figure 16. The tymbal cavity of a Tibicen male, with the tymbal cover and wings removed. Stippling indicates areas of thick sclerotized cuticle. Unstippled areas of the tymbal are thin sclerotized cuticle. Homopt26L.gif
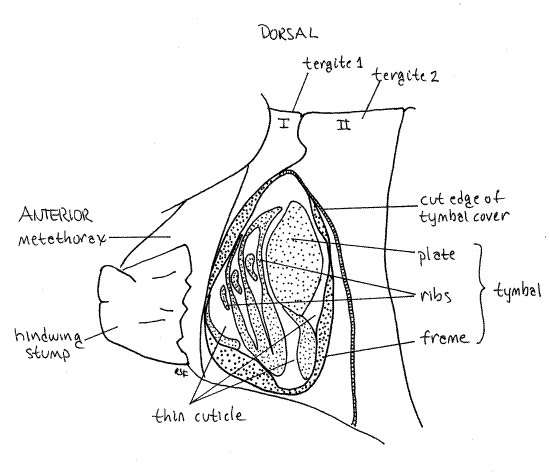
The tymbal is a complex cuticular structure consisting of several areas of alternating thin and thick sclerotized exoskeleton (Fig. 16). Most conspicuous is a thin area of flexible cuticle, the tymbal pad in which thickened tymbal ribs are embedded. The tymbal plate, and oval of more or less evenly thickened cuticle lies adjacent to the tymbal pad. A thickened cuticular rim, the tymbal frame, encloses the entire structure.
Large tymbal muscles in the abdomen insert on the tymbal and vibrate it to produce sound, which is amplified and modified by large air-filled resonating chambers in the metathorax and anterior five abdominal segments. The resonating chambers are expanded regions of the tracheal system.
Tympanum and Sound Reception
The opercula extending posteriorly from the metathorax cover the chordotonal organs (eardrums or tympanic membranes). Tympana are present in both sexes although they are larger in males.
From a specimen of either sex, use fine scissors to remove one of the opercula from the venter of the anterior abdomen. This will reveal a large auditory cavity, or chamber, whose posterior wall is a thin, smooth, roughly circular, cuticular tympanum. Vibrations in air cause the tympanum to vibrate in response and this movement is detected by sensory neurons attached to the tympanum.
Nymphs
External Anatomy
Living nymphs, being subterranean, are not often encountered but the empty exuviae of emerged individuals can often be found adhering to stems, sticks, and tree-trunks, which the nymphs have climbed after exiting the soil. Such exuviae are easily studied with a dissecting microscope and most features of nymphal external anatomy can be observed without recourse to intact specimens. Exuviae can be collected when available in the summer and stored in the laboratory stockroom until needed. The exercise is written so that it can be used independently of the imago exercise above, if desired.
Head
The body is divided into distinct head, thorax, and abdomen. The head is enclosed in a sclerified head capsule or epicranium, as is that of other insects (Fig 21-1A,B). Dorsally the capsule bears a pair of large, protruding compound eyes and a pair of slender filiform antennae, of 7-9 articles, depending on species (Fig. 17). Although small, the antennae are larger than those of the adult. The biarticulate peduncle of the antenna is partly overhung by the antennal shelf, a flattened ridge of the head capsule (Fig. 17, 18).
Ocelli are represented by inconspicuous spots in nymphs. The compound eyes, presumably useless to a subterranean animal, are opaque and white during most of nymphal life. Their corneas become transparent prior to the nymph's departure from the soil for the final molt to an aerial imago.
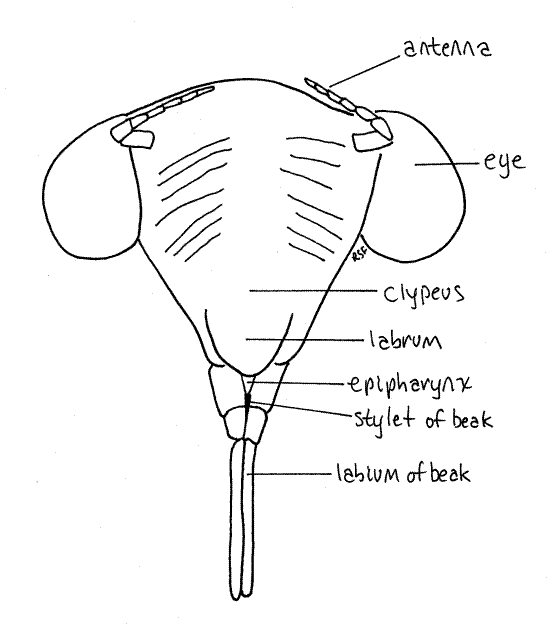
Most of the dorsal part of the head capsule, between the eyes, is the vertex (Fig. 19). The head capsule ventral to the eyes is the gena. A tiny triangle along the anterior edge of the vertex is bounded by inconspicuous sutures and is considered to be the frons by most authors (Fig. 18). Anteriorly, the face is formed of the large, conical, bulging, conspicuously striated clypeus, beginning along the ventral border of the frons (Fig. 20).
Figure 17. Side view of an unidentified cicada nymph. The midleg has been omitted for clarity. Homopt27L.gif
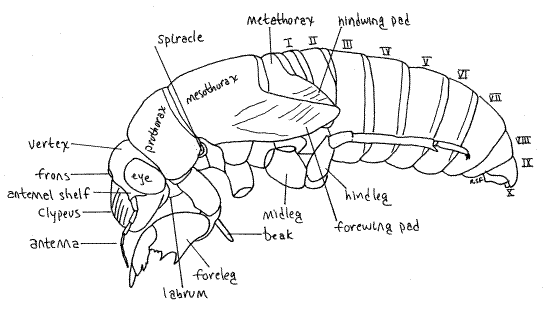
Figure 18. Dorsal view of the head of the final nymphal exuvium of the cicada, Tibicen. Homopt28L.gif
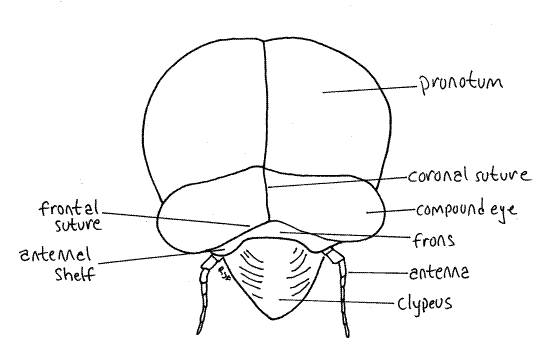
The mouthparts, consisting of highly modified mandibles, maxillae, and labium, form a beak (or rostrum) attached to the posterior, ventral border of the labrum (Fig 19, 20, 21-4). The beak extends away from the head between the bases of the forelegs. The labium forms a conspicuous grooved sheath of three distinct articles that encloses the much smaller, and quite inconspicuous (without dissection), black stylet. Most of the stylet is hidden inside the groove of the labium. The stylet is a cuticularized, hollow needle that is inserted in the roots of the host plant to remove fluid. It consists of long slender processes of the mandibles and maxillae and contains an outgoing channel for saliva and an incoming channel for food. It is the only part of the beak that is inserted into the host. The labium is a support and guide for the stylet but does not itself enter the prey.
Fig 19. Head and mouthparts of the final nymphal exuviae of the cicada Tibicen sp. A, Side view of the head and prothorax. The coxa and trochanter of the foreleg have been drawn as if transparent to reveal the base of the beak. B, The rostrum of the specimen in A. homopt29L.gif
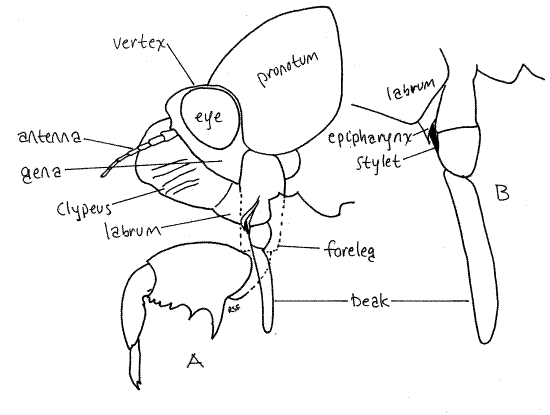
Figure 20. En face view of the head of the final nymphal exuviae of the cicada, Tibicen sp. Homopt30L.gif
Thorax
The thorax consists of three segments, prothorax, mesothorax, and metathorax. Each segment is covered by a dorsal sclerite. These are the pronotum, mesonotum, and metanotum, respectively (Fig 21-14). Of these, the prothorax, which houses the powerful muscles of the fossorial forelegs, is the largest. In contrast, the mesothorax, with the flight muscles of the forewings, is the largest thoracic segment of imagoes.
Each segment bears a pair of legs known, respectively, as forelegs, midlegs, and hindlegs. The forelegs of nymphs are large, powerful, multipurpose tools. Each consists of an enlarged, elongate coxa, a trochanter forming an "elbow", a massive femur, a finger-like tibia, and a biarticulate tarsus with two tarsal claws (Fig 17, 21). Adult tarsi, in contrast, are 3-articulate. The femur and tibia form a prehensile "hand" of which the tibia is a movable finger opposing the toothed palm of the femur. Foretarsi, which are absent in most instars, are present in the final instar for which their claws play an essential role in gripping bark when climbing prior to emergence. In some species the tarsus is folded away into a groove in the tibia until the final instar leaves the soil and is ready to climb a tree, at which time the tarsus is unfolded and its claws put to use.
Figure 21. Lateral view of the left foreleg of a final instar cicada nymph. Homopt31L.gif
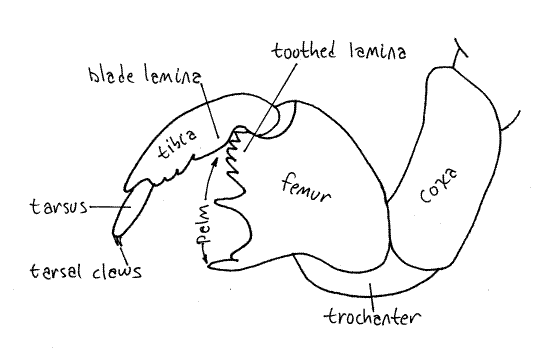
A smooth bladelike lamina at the base of the tibia opposes a toothed lamina of on the hinge end of the palm and presumably the two work together like scissors (Fig. 21). The toothed distal tip of the tibia opposes the toothed corner of the palm (of the tarsus) and together the two form a presumably prehensile organ used to hold roots close to the beak while feeding and also to carry soil during burrow and cell construction. The foreleg is a digging tool and its tibia is thrust into the soil like a pickaxe to loosen it. Functioning as a prehensile finger, it then opposes the palm and picks up this soil to pack it tightly against another wall of the cell. In this manner the shape of the cell is modified or a burrow constructed by moving soil from one wall to another. Nymphs also may construct chimneys or towers or cones of compacted pellets soil as much as 30 cm above the ground surface.
The midlegs and hindlegs are unmodified ambulatory legs consisting of a large proximal coxa, trochanter, femur, elongate tibia, and biarticulate tarsus with two distal tarsal claws (Fig. 17, 21-1E). The proximal tarsal article is much smaller than the distal one.
The mesothorax and metathorax each bear a pair of short, thick wingpads (Fig. 17, 21-13F). Upon emergence, the forewings and hindwings of the imago will develop from these.
Ten pairs of spiracles are present, two of them on the thorax and the remaining eight on the abdomen. The mesothoracic spiracle (spiracle 1) is located on the anterior border of the mesothorax under the posterior lateral corner of the pronotum (Fig. 17). Themetathoracic spiracle (spiracle 2) is on the anterior lateral metathorax at the base of the leg and under the wingpads. The spiracles are white.
The prothorax and mesothorax each have a large blunt midventral spine in some species.
Abdomen
The large abdomen consists of 10 segments (Fig. 17). Most segments have a large, sclerotized tergite arching over the dorsum and sides and extending onto the venter. A much smaller and flatter sternite covers the middle of the ventral surface (Fig. 22). Tergites and sternites are connected by flexible pleura. Segment 1 is small and is visible dorsally in the space between the wingpads. Segments 2-7 are similar is size and morphology. Segment 8 is smaller in diameter and tapers posteriorly. Segment 9 is much smaller and consists externally of a smooth dorsal tergite fitted over a smaller blisterlike ventral sternite. Segment 10 is tiny and extends posteriorly between the tergite and sternite of segment 9. It bears the terminal anus.
Figure 22. Ventral view of the abdomen of a cicada nymph. Homopt32L.gif
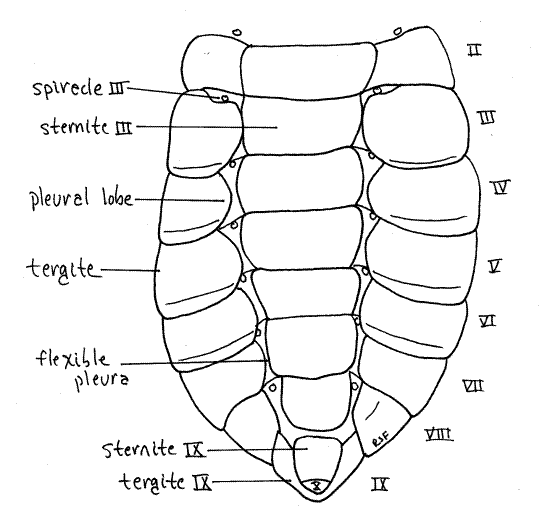
Sclerotized pleural lobes extend ventrally and medially from the lateral margins of the tergites to cover and protect the abdominal spiracles, presumably as an adaptation for life in the soil. The abdominal spiracles are white and located ventrally, on the anterior borders of segments 1-8 (spiracles 2-10), between the pleural lobes and the sternites (Fig. 22). They are smaller than the thoracic spiracles.
*Hyphenated figure call-outs, such as this one, refer to figures in Ruppert, Fox, and Barnes (2004). Those without hyphenation refer to figures embedded in this exercise.
References
Anon. Undated. Annual Cicadas of Arkansas (Tibicen spp). www.anglefire.com/ar/urobbie/tibicen.html
Borror DJ, Triplehorn CA, Johnson NF . An introduction to the study of insects, 6 th ed. Saunders College Publishing, Philadelphia. 875pp.
Century D. 2003. 220 Cicada Links. Cicada Mania and Dan Century. www.dancentury.com/cicada/cicadalinks.html
Chapman RF. 1998. The insects, Structure and function, 4 th ed. Cambridge Univ. Press, Cambridge. 769 pp.
Comstock JH. 1930. An introduction to entomology. Comstock Pub., Ithaca. 1044 pp.
Cooley J, Marshall D, O’Brien M. 2000. Cicadas of Michigan. University of Michigan Museum of Zoology. http://insects.ummz.lsa.umich.edu/fauna/Michigan_Cicadas/Michigan/Index.html
Gillott C. 1995. Entomology, 2 nd ed. Plenum Press, New York. 798 pp.
Heath JE, Hamilton WJ III. 1970. Temperature responses of the desert cicada, Diceroprocta apache (Homoptera: Cicadidae). Physiological Zoology 43:145-54.
Hickernell LM. 1920. The digestive system of the periodical cicada, Tibicen septendecim. Ann. Entomol. Soc. America, 13:223-242.
Myers JG. 1929. Insect singers, a natural history of the cicadas. George Routledge & sons, London. 304 pp.
Petti JM. 1997. University of Florida Book of Insect Records. Chapter 24 Loudest.
http://ufbir.ifas.ufl.edu/chapt24.htm
Ross HH. 1965. A textbook of entomology, 3 rd ed. John Wiley & Sons, New York. 539pp.
Ruppert EE, Fox RS, Barnes RB. 2004. Invertebrate Zoology, A functional evolutionary approach, 7 th ed. Brooks Cole Thomson, Belmont CA. 963 pp.
Snodgrass RE . 1935. Principles of insect morphology. McGraw-Hill, New York. 667 pp.
Toolson EC, Hadley NF . 1987. Energy-dependent facilitation of transcuticular water flux contributes to evaporative cooling in the Sonoran Desert cicada, Diceroprocta apache (Homoptera, Cicadidae). J. Experimental Biology 131:439-44.
Supplies
1 1iving, freshly collected, cicada
1 nymphal exuvium
1 small (sardine tin) wax-bottom dissecting pan
1 dissecting microscope
1 compound microscope (can be shared by several students or the entire class)
1 microscope slide and coverslip
1 microdissecting forceps
1 microdissecting (iridectomy) scissors
2 minuten nadeln with applicator stick handles
20 # 1 stainless steel insect pins
200 ml 0.7% saline solution
2 8-cm Carolina culture dishes or small jar
1 cotton ball
chloroform or ethyl acetate
1 plastic Pasteur pipet