Invertebrate Anatomy OnLine
Tetrastemma
with notes on Amphiporus, Zygonemertes, and Prosoma ©
Ribbonworms
30jun2006
Copyright 2001 by
Richard Fox
Lander University
Preface
This is one of many exercises available from Invertebrate Anatomy OnLine , an Internet laboratory manual for courses in Invertebrate Zoology. Additional exercises can be accessed by clicking on the links on the left. A glossary and chapters on supplies and laboratory techniques are also available. Terminology and phylogeny used in these exercises correspond to usage in the Invertebrate Zoology textbook by Ruppert, Fox, and Barnes (2004). Hyphenated figure callouts refer to figures in the textbook. Callouts that are not hyphenated refer to figures embedded in the exercise. The glossary includes terms from this textbook as well as the laboratory exercises.
Systematics
Nemertea P, Enopla C, Hoplonemertea O, Monostilifera sO, Tetrastemmidae F (Fig 11-13)
Nemertea P
Nemertean worms are active, benthic predators that use an eversible, sticky or barbed, and sometimes poisonous proboscis to capture prey (Fig 11-4). Nemerteans are long and slender, aptly known as ribbon worms, or rubber band worms. The longest is about 50 m but most are much less than that, usually no more than 20 cm. Many are brightly colored. Most of the 1150 species are marine but a few live in freshwater or terrestrial habitats.
The gut is complete and extends from anterior mouth to posterior anus. The long proboscis is housed in a cavity, the rhynchocoel, from which it is everted by hydrostatic pressure generated by surrounding muscles. The tubular proboscis everts, by turning inside out, from an anterior proboscis pore which may or may not be independent of the mouth.
An anterior brain and a pair of longitudinal lateral nerve cords are present. Excretion is via protonephridia. Nemerteans are gonochoric. The body wall is complex with numerous layers of muscles, epithelia, spaces, and connective tissue, and varies with order. There is no cuticle.
Nemerteans have traditionally been thought of as having a compact body plan because the viscera do not appear to be enclosed in a body cavity. Recent studies however have revealed the presence of a coelom and necessitate the reevaluation of the phylogenetic affinities of these animals. The fluid transport system has been shown to be a coelomic space and the rhynchocoel is also a coelomic space.
Enopla C
The nerve cords are internal to the body wall muscles. The mouth is anterior to the brain. The proboscis is armed in most.
Hoplonemertea O
These, the armed nemerteans, have the proboscis tipped with one or more stylets.
Monostilifera sO
The tip of the proboscis bears a single stylet on a bulbous base. When the proboscis is everted, the calcareous stylet is exposed and in position to penetrate the prey. The four genera supported by this exercise belong to this taxon.
Laboratory Specimens
Nemerteans can be studied in the laboratory by dissecting very large specimens or by examining very small ones with the compound microscope. Sufficient numbers of large individuals are rarely available, either alive or preserved, for use by an entire class and small species are preferred for that reason. Unfortunately small living nemerteans are usually available only at coastal locations.
Small worms belonging to the marine genera Tetrastemma (Tetrastemmatidae), Amphiporus (Amphiporidae), or Zygonemertes (Amphiporidae) and the freshwater Prostoma (Tetrastemmatidae) are similar and any of these can be used for this study. All are monostyliferans. Individuals of these genera are small enough to be mounted on a glass slide and examined whole with the compound microscope. The exercise is written specifically for living, relaxed Tetrastemma but, for the purpose of this study, the four genera are nearly identical anatomically and can be used interchangeably. Another exercise in the OnLine Invertebrate lab manual is written for Cerebratulus, a large unrelated species.
External Anatomy
Place a living worm in a small culture dish of seawater (or pondwater if your worm is Prostoma). Put the dish on the stage of the dissecting microscope and observe the behavior of the animal. Locomotion, via ciliary gliding, is rapid and effective. These small nemerteans are nearly cylindrical with only a modest amount of dorsoventral flattening.
The animal is worm-shaped, being long and narrow (Fig 1). It is slightly depressed dorso-ventrally. The head is the anterior end. Tetrastemma and Prostoma rarely exceed 3 cm in length but Amphiporus and Zygonemertes are often larger.
Internal Anatomy
If you have one of the marine species, transfer it to a drop of magnesium chloride on a microscope slide and watch as it quickly relaxes. (If you have a freshwater worm, relax it in carbonated water, pondwater saturated with chloroform, or 5% ethanol in pondwater.) Orient the relaxed worm on the slide so its ventral side is down. The black ocelli on the head are dorsal and can be used as landmarks.
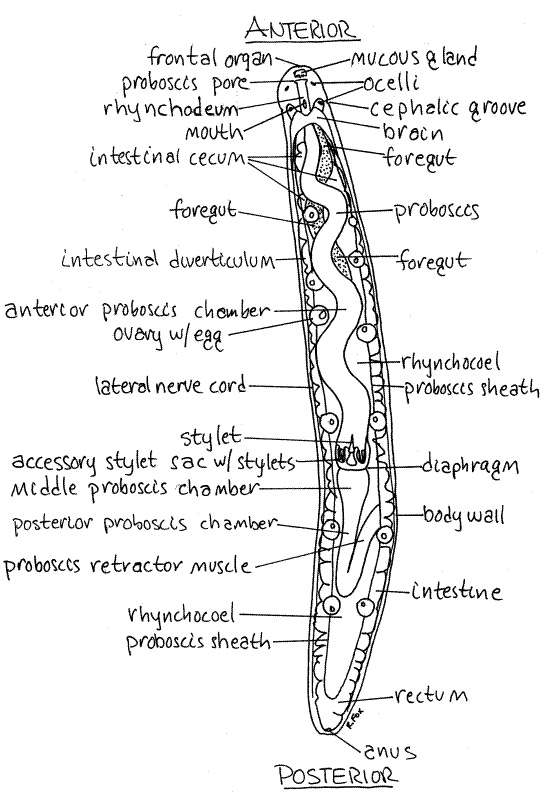
Figure 1. A female Tetrastemma from Coos Bay, Oregon in dorsal view. The excretory, posterior nervous system, and coelomic transport systems are omitted. Nemertea5La.gif
Apply small wax feet to the four corners of a coverslip (see Techniques chapter) and place it over the worm. Be sure the worm is near the center of the coverslip as it may continue to move even though anesthetized (magnesium chloride has no effect on cilia). Gently press the corners of the coverslip to flatten the wax so the coverslip touches the back of the worm. Apply a little more pressure to each corner in turn until the worm is slightly squeezed. Blot the excess liquid from the edges of the coverslip. You can adjust the thickness of the worm by applying or removing pressure to or from the coverslip (add more liquid to lift the coverslip, press on the wax feet to lower it). If you squeeze too much, the worm will be distorted and may rupture. If you attempt to squeeze the worm before it is fully relaxed, it is likely to evert its proboscis. Transfer the wholemount to the stage of the compound microscope and study it using 40X and 400X with carefully adjusted light. If there are extra worms, squeeze a worm deliberately to make it extend its proboscis.
Nervous System and Sense Organs
Look at the head of the worm (Fig 2). The brain consists of dorsolateral ganglionic masses of nerve cells connected above and below the gut and proboscis by transverse connectives to form a nerve ring. The outline of the brain is easily seen at the posterior end of the head. Extending posteriorly from its posterolateral corners are two large lateral nerve cords, one on each side. They can be seen lying adjacent to the thick body wall (Fig 1, 2, 11-5A).
Figure 2. The anterior end of the monostyliferan Tetrastemma. Nemertea6L.gif
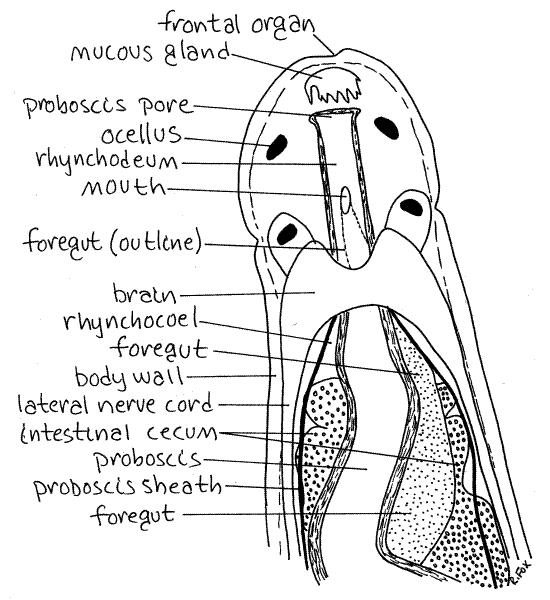
Identify the black eyespots, or ocelli on the dorsal side of the head. Their arrangement is characteristic of the genus. Tetrastemma has four ocelli arranged in a trapezoidal or quadrangular configuration (Fig 2). Zygonemertes has numerous ocelli scattered randomly over the head and extending posteriorly in a row on each side. Amphiporus has two short rows of ocelli, one on each side of the head and confined to the head (Fig 11-9B*). Prostoma has two short rows, usually consisting of 3 pairs of ocelli, on the head.
Two ciliated chemosensory cephalic grooves are situated on the ventral surface of the head. The two grooves form a "V" with its apex pointed anteriorly (Fig 11-9B). Its arms reach the sides of the head where they appear in dorsal view as small ciliated dimples (Fig 2). If your animal happens to be mounted upside down, the cephalic grooves will be easy to see. In living animals the activity of the cilia in the dimples or grooves is visible. Chemosensory organs in invertebrates are often ciliated pits, grooves, or tubes.
In addition, a pair of ciliated pits, the cephalic organs, is situated beside the brain and opening to the exterior laterally, near the ends of the cephalic grooves. You may not see these.
A ciliated frontal organ is located at the anterior tip of the head. It is an inconspicuous dimple (Figs 1, 2). Associated with it is a cephalic gland (mucous gland) consisting of a cluster of pigmented (white) secretory mucus gland cells. The cephalic gland is a conspicuous white spot but will appear black under the transmitted light of your compound microscope.
Digestive System
In hoplonemerteans the proboscis and gut share a common external opening, the proboscis pore. The proboscis pore of Tetrastemma is immediately posterior to the tip of the head on the ventral midline and is the external opening of the rhynchodeum (Fig 2, 11-5B). The rhynchodeum is a common chamber into which both foregut and proboscis open. The opening of the gut into the rhynchodeum is the mouth. Note that the mouth is anterior to the brain, a defining characteristic of Enopla. In many nemerteans (e.g. Cerebratulus) the proboscis pore and gut open at the body surface independently of each other (Fig 11-2B). In these worms the gut does not join the rhynchodeum.
The gut is divided into foregut and intestine (Fig 11-5A). The foregut is difficult to recognize in squeeze preparations even though it is large and easily seen once you know what it looks like. It is a curved, thick tube bulging to the side posterior to the brain (Figs 1, 2). It runs far posterior in the worm before joining the midgut, or intestine, which is ventral to it. All you can see of the foregut are parts of its outline where it is not obscured by the proboscis and proboscis sheath.
The intestine is large and dark. Its sides bear numerous short, wide, conspicuous, lateral intestinal diverticula that extend to the body wall and fill most of the space in the interior of the worm (Figs 1, 3).
The foregut joins the intestine at a point well posterior to the anterior end of the intestine. As a consequence, the anterior end of the intestine is a large blind sac, the intestinal cecum (Figs 1, 2) . (The foregut of Cerebratulus opens directly into the anterior end of the intestine and there is no cecum.)
Far posteriorly, the intestine narrows to become the hindgut, or rectum, which opens via the anus at the posterior end of the worm (Fig 1).
Proboscis and Proboscis Sheath
The proboscis itself is easily seen, in fact it is one of the most obvious structures in the animal (Fig 1, 11-5A). It begins anteriorly at the atrium and extends posteriorly, coiled and twisted, for about ¾ of the length of the worm. It is a hollow tube that can be everted by turning inside out through the proboscis pore/mouth. The lumen of the proboscis is the proboscis chamber (Figs 1, 2, 3).
The proboscis is contained in a long, wide coelomic cavity, the rhynchocoel, which should be visible (Figs 1, 2, 3, 11-5A,B). The wall of the rhynchocoel is the proboscis sheath (Figs 1, 2, 3). Contractions of muscles in the rhynchocoel wall elevate fluid pressure in the rhynchocoel and evert the proboscis.
The proboscis is divided into the anterior proboscis and the posterior proboscis by a transverse diaphragm (Fig 1, 3). Only the anterior proboscis is everted. Most of the posterior third is a retractor muscle whose origin is the posterior proboscis sheath . The retractor is stretched when the proboscis is everted and its contraction pulls the proboscis back into the rhynchocoel (Fig 1).
Figure 3. The midbody region of the hoplonemertean Tetrastemma. Nemertea8La.gif
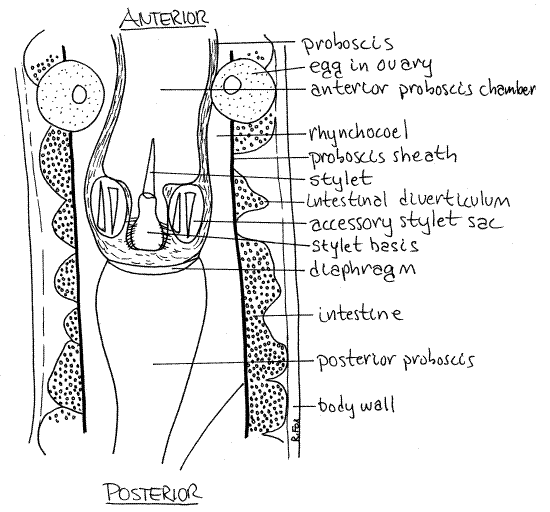
The lumen of the eversible portion is the anterior proboscis chamber (Fig 1, 3). That of the non-eversible region is the posterior proboscis chamber. Poison is secreted and stored in the posterior, noneversible portion of the proboscis, is used to kill or stun the prey.
In Hoplonemertea the proboscis is armed with a hard calcareous stylet used to stab the prey to facilitate the entry of the toxin into the wound (Fig 1, 3, 11-5C,D, 11-6). When the proboscis is everted, the stylet is at the tip (Fig 11-3C). Reserve stylet sacs containing reserve stylets (= accessory stylets) can be seen beside the currently functional stylet (Fig 3, 11-5C). Reserve stylets are spares to replace stylets lost during feeding. The stylet is attached to the proboscis wall by a bulbous stylet basis.
Reproductive System
Nemerteans are gonochoric and the numerous paired gonads are large sacs located laterally between the intestinal diverticula (Fig 11-5A). Each opens independently to the exterior but the openings will probably not be seen. Mature eggs are easily recognized in ovaries (Fig 1). There is one egg per ovary.
Fluid Transport Systems
Nemerteans have a fluid transport system composed of vessels extending through the body but unless you happen to have a specimen of Amphiporus cruentatus, you will not see these vessels (Fig 11-7B). This species, which occurs on both coasts of North America, has bright red hemoglobin in the vessels which make them easy to see in living specimens. The channels look like blood vessels but are coelomic, not hemal.
The system consists of a pair of lateral longitudinal vessels, a dorsal longitudinal vessel, and anterior and posterior connections between them (Fig 11-7B). The vessels are contractile but, unlike blood vessels, they are lined by a ciliated mesothelium. The vessels are now believed to be coelomic spaces rather than blood vessels. That the hemoglobin in these vessels is cellular is consistent with this hypothesis. You may remember that invertebrate hemoglobin is almost always corpuscular in the coelom but in solution in the hemal system.
The rhynchocoel functions as a local fluid transport system supplying the proboscis with nutriment. It is also coelomic space.
Excretory System
Two or more protonephridia, each with multiple terminal cells, drain by ducts to nephridiopores located laterally in the region of the foregut. The protonephridia are not apparent in these preparations. The terminal cells of the protonephridia bulge into the lateral coelomic vessels (Fig 11-8) and are important osmoregulatory organs.
Lab Prep
Collection of Marine Specimens
Sufficient numbers of Tetrastemma, Amphiporus, and Zygonemertes can usually be collected from shallow-water marine habitats using an "environmental deterioration" technique.
Potential substrata such as oyster clumps, encrusted rocks, or algal mats are placed in a bucket of sea water in the laboratory. Do not aerate the water, rather allow the oxygen tension to drop as bacteria and animals respire over a period of several hours. Many small worms, including polychaetes, turbellarians, and nemerteans will slowly crawl up the sides of the bucket to the air-water interface where they can be harvested with a fine forceps or pipet and transferred to a small culture dish of clean, oxygenated seawater. The threadlike worms are mostly less than 3-4 cm in length and 1 cm is ideal for this study. Amphiporus can be purchased from the Woods Hole Marine Biological Laboratory.
Collection of Freshwater Species
The freshwater species, Prostoma rubrum, can sometimes be collected locally and cultured in the laboratory thus facilitating the study of living nemerteans at inland locations. This animal is widely distributed in North America but its occurrence is not uniform. It is most common in the fall, in clean still or slow moving water in filamentous algae or rooted plants. It reportedly can be maintained in the laboratory in well-aerated aquaria by feeding it nematodes, small crustaceans, chopped liver, or oligochaetes.
References
Goodchild CG. 1950. Amphiporus ochraceous, pp 209-214 in Brown FA (ed). Selected Invertebrate Types. Wiley, New York. 597p.
Coe WR. 1895. Anatomy of a species of nemertean (Cerebratulus lacteus Verrill) with remarks on certain other species. Trans. Connecticut Acad. Sci. 9:479-514, pls. 10-15.
Coe WR. 1905. Nemerteans of the west and northwest coasts of America. Bull. Mus. Comp. Zool, Harvard 47:1-320, pls. 1-25.
Coe WR. 1937. Methods for the laboratory culture of Nemertea. pp.162-165 in Needham JG. et al. Culture Methods for Invertebrate Animals. Comstock, Ithaca.
Coe WR. 1943. Biology of the nemerteans of the west coast of North America. Trans. Conn. Acad. Arts Sci. 35:129-328, pls. 1-4.
Pennak RW . 1989. Fresh-water Invertebrates of the United States, 3 rd ed. Wiley, New York. 628p.
Ruppert EE, Fox RS. 1988. Seashore animals of the southeast. Univ. South Carolina Press, Columbia, 429.
Ruppert EE, Fox RS, Barnes RB. 2004. Invertebrate Zoology, A functional evolutionary approach, 7 th ed. Brooks Cole Thomson, Belmont CA. 963 pp.
Turbeville JM. 1991. Nemertinea, pp. 285-328 in Harrison FW, Bogitsh BJ (eds.). Microscopic Anatomy of Invertebrates vol. 3 Platyhelminthes and Nemertinea . Wiley-Liss, New York.
Turbeville JM, Ruppert EE . 1985. Comparative ultrastructure and the evolution of nemertines. American Zool. 25-71.
Supplies
Compound microscope
Dissecting microscope
Slides and cover glasses
Living nemertean
Seawater (or pondwater if using Prostoma)
Isotonic magnesium chloride