Invertebrate Anatomy OnLine
Strongylocentrotus drobachiensis ©
Green Sea Urchin
25may2007
Copyright 2001 by
Richard Fox
Lander University
Preface
This is one of many exercises available from Invertebrate Anatomy OnLine , an Internet laboratory manual for courses in Invertebrate Zoology. Additional exercises, a glossary, and chapters on supplies and laboratory techniques are also available at this site. Terminology and phylogeny used in these exercises correspond to usage in the Invertebrate Zoology textbook by Ruppert, Fox, and Barnes (2004). Hyphenated figure callouts refer to figures in the textbook. Callouts that are not hyphenated refer to figures embedded in the exercise. The glossary includes terms from this textbook as well as the laboratory exercises.
Systematics
Echinodermata P, Eleutherozoa, Cryptosyringida, Echinozoa, Echinoidea C, Euechinoidea sC, Echinoida O, Strongylocentrotidae F (Fig 9-26, 27-12, 28-62)
Echinodermata P
Echinoderms are secondarily radially symmetric deuterostomes whose ancestors were bilaterally symmetric. The adult radial symmetry is pentamerous with body parts occurring in fives or multiples thereof. Echinoderms have strong affinities with the ancestral trimeric deuterostomes especially in the tripartite organization of the coelomic cavities. Echinoderm larvae have the coelom divided into three regions, as is typical of the early coelomates, and these regions have important adult derivatives. All echinoderms are marine and benthic. About 6000 Recent species are known but the fossil record includes 13,000 extinct species.
An important echinoderm apomorphy is the water vascular system that in most groups functions in support of locomotory tube feet but is also important in gas exchange, excretion, and feeding. The body wall includes a thick connective tissue dermis in which calcareous ossicles (little bones) are almost always an important component. These ossicles make up an endoskeleton which assumes different forms in different taxa. In most echinoderms calcareous spines of various sizes and shapes arise from the dermis and extend from the body surface and are alluded to by the name echinoderm (= spiny skin). The connective tissue is mutable and its consistency is under nervous control.
Excretion in echinoderms is accomplished by simple diffusion of metabolic wastes (ammonia) across thin permeable regions of the body wall. A variety of gas exchange structures, including the tube feet, is found in various echinoderms. A hemal system is present but its role in transport is still poorly understood and the chief transport system is the circulating fluid of the various coelomic compartments. The hemal system may be through transport system that delivers nutrients from the gut to these compartments for local distribution. The nervous system consists of two central intraepidermal nerve rings from which arise radial nerves to the periphery. Echinoderms are gonochoric and fertilization is usually external.
Eleutherozoa
Eleutherozoans are mobile echinoderms in which the oral surface is oriented against the substratum. A madreporite and locomotory tube feet are present. Polian vesicles and Tiedemann’s bodies may be present on the ring canal. Movable spines are present. Eleutherozoa includes all Recent echinoderms except for its sister taxon, Crinoidea.
Cryptosyringida
Cryptosyringida includes Ophiuroidea, Echinoidea, Holothuroidea, all with closed ambulacra in which the radial nerve is internalized and protected by ossicles (Fig 28-20).
Echinozoa
Echinozoa, sister taxon to Ophiuroidea, consists of the urchins and sea cucumbers. In these echinoderms the oral surface and ambulacra have expanded aborally until they enclose almost the entire body except for a small aboral periproctal region with the anus. A bony ring of ossicles surrounds the pharynx. The hemal system is better developed than in other echinoderms. The tube feet have ossicles
Echinoidea C
Echinoidea includes about 950 extant species of sea urchins, sand dollars, sea biscuits, heart urchins, and their relatives. The dermal ossicles are thin plates fused to form a rigid, more or less spherical, endoskeletal test. Except for a thin outer epidermis, all the soft anatomy is inside the test. The larva is a bilaterally symmetrical echinopluteus. The test is covered by an abundance of movable spines. Tube feet are the major respiratory organs and the madreporite is aboral.
Urchins may be regular or irregular. Regular urchins are the sea urchins, with radial symmetry, globose nearly spherical bodies, and long spines. Most are epifaunal. Irregular urchins are sand dollars, sea biscuits, and keyhole, heart, and cake urchins. These are usually infaunal in soft sediments and have a superficial bilateral symmetry superimposed on their radial symmetry. The body is usually flattened and the spines short.
Epifaunal species (regular urchins) possess a feeding apparatus known as Aristotle's lantern, equipped with five strong teeth, used for scraping food from hard substrates. The lantern is reduced in infaunal species (irregular urchins) because most of them are deposit feeders.
Laboratory Specimens
Strongylocentrotus, is a taxon of common regular urchins often used in invertebrate zoology courses to exemplify echinoid anatomy. This exercise is written specifically for the green sea urchin, Strongylocentrotus drobachiensis, but could also be used for any other species of Strongylocentrotus, as well as Arbacia, Lytechinus, or Echinus. Strongylocentrotus drobachiensis occurs on the northeast and northwest coasts of North America and in Europe. Strongylocentrotus franciscanus and S. purpuratus occur on the North American Pacific coast from Alaska to Baja California. Use the dissecting microscope as needed to study a living or preserved specimen. Living specimens should be immersed in seawater for the beginning of the exercise and then transferred to magnesium chloride prior to dissection. Preserved animals should be in tapwater.
External Anatomy
General
Examine a living or preserved regular urchin. Strongylocentrotus, like most regular urchins, is a spheroid with one flattened pole (Fig 28-36). The lower, oral pole, is flattened whereas the opposite,aboral pole, is more rounded. The animal is pentamerously radially symmetrical. The two poles are connected by the aboral-oral axis, which is the axis of symmetry. Structures remote from this axis are said to be peripheral whereas those near the axis are central. In radially symmetrical animals the terms anterior-posterior, dorsal-ventral, right-left are irrelevant and have no meaning. Five meridional ambulacral axes extend over the surface of the sphere from oral to aboral poles.
The surface of the body is firm and rigid due to the underlying internal test of fused calcareous ossicles. The test is hollow and most of the animal's soft parts are inside it. The test is an endoskeleton, however, and is located in the connective tissue dermis of the body wall and covered by a thin, inconspicuous, ciliated epidermis. Because the epidermis is not readily apparent, the test appears to be external.
Spines
Most of the surface bears articulated, movable spines, which are also part of the connective tissue skeleton and are also covered by a thin epidermis. The spines of S. drobachiensis have longitudinal ridges and grooves and are pointed at the apex, wider at the base. Strongylocentrotus drobachiensis has large primary and smaller secondary spines, of similar shape. (Some urchins have spines of different shapes and in some the spines are all approximately the same size. Strongylocentrotus purpuratus has some small cylindrical spines in addition to the larger conical ones. Arbacia punctulata has primary but no secondary spines.)
The basal end of each spine bears a socket that articulates with a ball, or tubercle, on the test (Fig 28-32A, 28-34). A ball and socket joint permits motion in a wide range of directions. An outer ring of muscles and an inner ring of collagen fibers extend from the spine to the test (Fig 28-32A). Selective contraction of muscles in the outer sheath move the spine in any desired direction atop its tubercle. The collagen fibers of the inner sheath can be locked so that the spine is fixed rigidly in position. So effective is this locking mechanism that the spines cannot be moved without breaking them.
>1a. Pull a large spine from the test. Look at the center of its base for the socket. With magnification examine its tubercle on the test. <
>1b. If your specimen is living, touch one area of its epidermis with a needle and watch the response of the nearby spines. <
Tube Feet
The 10 meridional rows of long slender tube feet, or podia, extend between the oral and aboral poles. These rows are said to be meridional since they run from pole to pole as do meridians, or lines of longitude, on a globe. The tube feet of regular urchins are normally much longer than the spines but those of stressed or preserved animals are contracted and shorter.
The 10 rows of tube feet are arranged into five ambulacra consisting of two rows of tube feet each (Fig 1, 28-30B). The ambulacra are separated by zones without tube feet, known as interambulacra, of which there are five also. This arrangement of rube feet is difficult to discern in intact whole animals but you will see it clearly when you study a cleaned test.
Most tube feet end in wide suckers used to hold the animal firmly to hard substrata. The tube feet are used for locomotion and respiration, and some urchins, such as Lytechinus variegatus, use them to hold bits of shell or vegetation above the body, presumably for camouflage or protection from UV radiation in shallow water (Fig 28-39). The behavior is known to be a response to high light intensities. The tube feet of the aboral hemisphere of Arbacia are mostly suckerless and have a sensory role.
The sucker is reinforced with tiny flat ossicles that help it resist deformation under stress (Fig 28-37A). Deformation of the sucker, if it were allowed to occur, would result in loss of suction. In addition, slender curved ossicles, resembling the spicules of sponges, are present in the tube foot to support its walls.
>1c. Remove a suckered tube foot and place it on a slide in a drop of bleach. Set it aside until the soft tissue has been oxidized and bubbles cease to form and then affix a coverslip. Examine the ossicles with the compound microscope and consider their arrangement in an intact podium. Note that the flat ossicles are perforated by tiny pores. The pores impede the propagation of cracks in the ossicle and are typical of many echinoderm ossicles. <
Peristome
The center of the oral surface is free of spines and is not underlain by skeleton, there being a large hole in the test at this position. This region is the peristome (peri = around, stome = mouth) and the hole in the test is the peristomial aperture. You cannot see the aperture now because it is covered by the soft peristomial membrane with the mouth at its center (Fig 28-30A, 28-36).
The triangular white tips of the five teeth of Aristotle's lantern can be seen protruding from the mouth (Fig 28-30A). The mouth opens into the pharynx, which cannot be seen at present. Five pairs of short, thick tube feet, called buccal podia, are arranged in a circle on the peristomial membrane around the mouth. Five pairs of large bushy peristomial gills are hidden in the spines around the margin of the peristome. They may be difficult to see in the forest of spines, especially in preserved specimens.
>1d. If your specimen is alive, give one of the buccal podia a gentle pinch and watch the response of the neighboring pedicellariae. <
Numerous pedicellariae (singular = pedicellaria) of several types are present on the body surface and some can be seen in the vicinity of the mouth (Fig 28-34). Echinoid pedicellariae have three tiny jaws at the end of a pedicle. Pedicellariae have a skeleton consisting of three ossicles in the jaws and a long slender ossicle in the pedicle.
>1e. Remove a pedicellaria and make a wet mount. Examine the preparation with the compound microscope and find the calcareous jaws and the calcareous rod in the pedicle. <
Periproct
The periproct is a small region at the aboral pole surrounding the anus. The periproct is much smaller than the peristome and, since it is surrounded by the spines of the aboral hemisphere, is harder to see. The periproctal aperture through the test is covered by the periproctal membrane, which in Strongylocentrotus is underlain by numerous tiny calcareous ossicles (Fig 1, 28-31A). (In Arbacia it is covered by four large, conspicuous ossicles, the anal plates.) The ossicles of Strongylocentrotus are not fused together, however, and the membrane is flexible. The periproctal aperture cannot be seen in intact specimens. Use a strong jet of water from a pipet or squirt bottle to wash the mucus from the aboral pole.
The periproct is at the exact center of the aboral surface but the anus is a little off center, near one side of the periproct. (In Arbacia the anus is at the pole.) The anus is a large opening on a slight elevation and is surrounded by an irregular array of small spines. The elevation bears numerous tiny, spiny knobs.
The periproct is surrounded by a ring of five large ossicles, the genital plates, and five smaller ocular plates that are part of the rigid test and are fused to it (Fig 1, 28-31A). These cannot usually be seen in whole specimens, as they are covered by the epidermis and spines. You will find them later when you study a cleaned test.
One of the genital plates is modified to form a madreporite and this one is visible externally (Fig 1, 2, 28-31A). The large, triangular madreporite lies to one side of the periproct on an interambulacral axis. Its surface bears obvious perforations and is covered by a ciliated epithelium. The madreporite is penetrated by numerous ciliated channels continuous with the stone canal and axial canal of the water vascular system in the interior.
The five genital plates, including the madreporite, each bear an opening, the gonopore, through which a gonoduct opens. Although it may not be visible, the gonopore of the madreporite can be found by probing the apex of the madreporite with a microneedle. The needle will slip into the pore and pass completely through the test. Gonopores will be much easier to see later when you examine a cleaned test. The gonopores are interambulacral.
>1f. Gametes may sometimes be released from the gonopores of living specimens while you watch. Eggs are orange, sperm are white. If this happens, make a wetmount of the gametes (using seawater and a wax-supported coverslip) and examine it with the compound microscope. Ova are large immobile spheres whereas sperm are tiny, rapidly moving flagellated cells. Watch the ova as they are transported by ciliary currents of the epidermis. <
>1g. If gametes are available, mix eggs and sperm in a dish of clean seawater. Prepare a wetmount of the eggs using a supported coverslip, and observe with the compound microscope at 100 and 400X. Unfertilized eggs are simple spheres but zygotes have a distinctive, raised fertilization membrane around the periphery. The membrane is a barrier to the entry of superfluous sperm. Note the numerous sperm caught in the membrane. Note also the enormous disparity in size between eggs and sperm. Make additional wetmounts from the dish later in the period. Cleavage should begin in about 1-2 hours. <
Internal Anatomy
If your animal is alive, replace the seawater in the dish with isotonic magnesium chloride and set the dish aside while you study a cleaned test.
Test
Although it appears to be external, the rigid test is part of the dermis and is covered by a thin epidermis. Study a cleaned test from which all soft parts have been removed by oxidation with bleach. Ideally the tiny ossicles of the periproct should be present and intact, although in commercially prepared tests they rarely are.
The test is composed of 20 meridional rows of thin calcareous ossicles, or plates (Fig 1, 28-30B). The rows are easiest to see from the inside. Hold the test against a white background and look into the peristome. The plates and podial pores should be obvious. The plates are homologous to the dermal ossicles of other echinoderms and differ from them primarily in that they are thin, platelike, and fused together. They form a rigid endoskeketon whose articulations are fixed and immovable. Because the test is rigid, the rest of the body wall is poorly developed and lacks the thick layer of connective tissue and muscles characteristic of other echinoderms.
The 20 rows of plates are organized into five ambulacra alternating with five interambulacra around the circumference of the test. Each ambulacrum consists of two rows of ambulacral plates and the interambulacra consist of two rows of interambulacral plates (Fig 1, 28-30B). The ambulacral plates are easily recognized because each one bears several pairs of perforations for the tube feet, known as podial pores. Each pair of pores serves one tube foot (Fig 28-37A). The interambulacral plates do not bear pores.
The meridional line running along the center of an ambulacrum, between its two rows of tube feet, is an ambulacral axis, or radius. Similarly, the meridian running along the midline of each interambulacrum is an interambulacral axis, or interradius.
In regular urchins, such as Strongylocentrotus, the periproct is at the aboral pole and is the aboral ambulacral center on which the ambulacra converge. Similarly, the peristome at the oral pole is the oral ambulacral center , from which the ambulacra radiate.
Examine the aboral surface with moderate magnification (10-15X) and observe the ambulacral plates and their articulations with adjacent plates. Examine the shiny, rounded tubercles, which in life articulate with the sockets of spines (Fig 28-31B). The tubercles sit atop low mounds on the surface of the plates. The muscles that operate the spines originate on the slopes of the mounds and insert on the bases of the spines (Fig 28-32A).
Find the large peristomial aperture at the oral pole. In life this opening is covered by the peristomial membrane and the mouth lies in its center (Fig 28-30A). Five calcareous protrusions, the auricles, are arranged on the ambulacra around the inside margin of the aperture (Fig 3). The auricles are sites for origin of the muscles that operate Aristotle's lantern and each is an arch through which the radial canal and radial nerves pass. The auricles are good landmarks for quickly locating the ambulacral axes.
Turn your attention to the aboral pole and find the periproctal aperture at the aboral pole. In life this aperture is covered by the periproctal membrane in which are embedded many very small, loosely articulated periproctal plates (Fig 1, 28-31A). The membrane and plates may be missing from your dried test and, if so, a large hole is present instead.
Figure 1. The aboral pole of a cleaned Strongylocentrotus test. Echinoid10L.gif
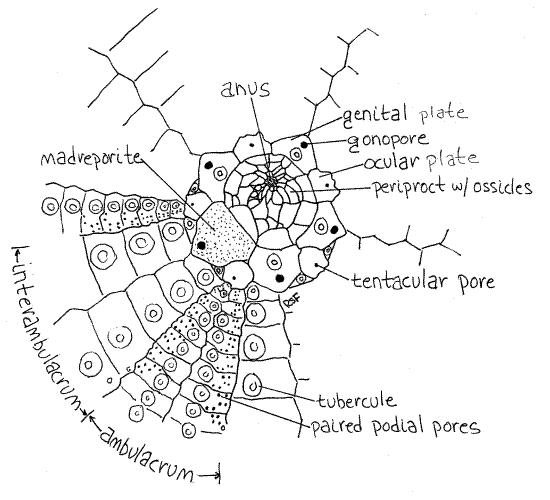
A ring composed of 10 ossicles, of two types, surrounds the periproctal aperture and each bears a small pore. Five smaller ossicles lie on ambulacral axes and are ocular plates (Fig 1, 28-31A). Each bears a small tentacular pore for the emergence of the end of a radial canal.
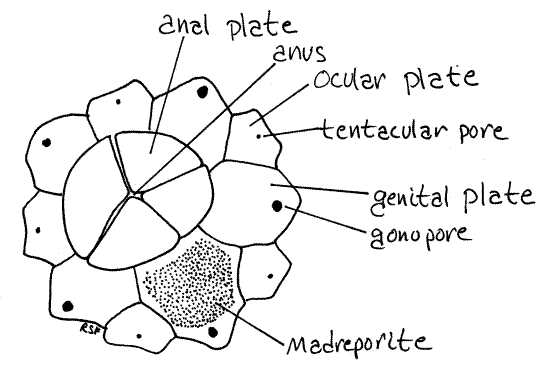
Five additional ossicles, the larger genital plates, alternate with the ocular plates and lie on interambulacral axes (Fig 1, 28-31A). Their pores are gonopores, which are larger than the tentacular pores. One of the genital plates is much larger than the others and is the madreporite. Look at its outer and inner surfaces with magnification and observe that it is perforated by numerous closely spaced canals. (In Arbacia four large anal plates at the aboral pole replace the profusion of tiny ossicles characteristic of Strongylocentrotus (Fig 2). In life the anal plates open like trapdoors to expose the anus. The genital plates of Arbacia are easily seen in whole specimens. Like the smaller ossicles of Strongylocentrotus, the four anal plates are usually lost in the preparation of dry skeletons.)
Figure 2. Aboral pole and ossicles of Arbacia. Echinoid43L.gif
Soft Anatomy
You should now reveal the viscera by removing the test from the aboral hemisphere of your specimen. The organs should remain undisturbed in the cup formed by the test of the oral hemisphere. The object is to remove the aboral hemisphere of the test while leaving the periproct intact, but unsupported, with the rectum, stone canal, and axial complex still attached to it as in Figure 3. The axial complex is composed of the axial hemal vessel (= axial gland), axial canal (= axial sinus), and associated structures. The axial hemal vessel is part of the hemal system whereas the stone canal is part of the water vascular system. The axial canal is a coelomic space continuous with the stone canal and madreporite. These will be discussed later.
" Force the sharp point of a pair of heavy scissors through the edge of the periproct opposite the madreporite. Make a meridional cut through the body wall, including the test, almost to the equator (widest part of the body). It will help if you remove the spines from the areas you intend to cut. Carefully separate any broken pieces of test from the underlying soft tissue and remove them. When you get almost to the equator turn the cut 90 º in either direction and make a latitudinal (equatorial) cut completely around the circumference until you have completed a circle.
Return to the aboral pole and cut a second circle around the periproct and madreporite. Your task now is to remove all of the test between the two circular cuts, i.e. most of the aboral hemisphere. Do this piecemeal, by breaking away pieces of the test. Lift the edge of the meridional cut, look inside, and VERY CAREFULLY use a teasing needle (not minuten nadel) free the adhering tissue from the inner wall of the test. Most of this tissue is gonad and gut and must be carefully separated from the test. The walls of the gut are very delicate and tear easily. Under each row of tube feet is a meridional row of soft, low ampullae that will remain with the test. Do not try to separate the ampullae from the test.
As you free large areas of the test from the adhering tissue, break that part off and set it aside. Proceed to each successive area until you have removed the entire aboral hemisphere with the exception of the periproctal region. The stone canal and the rectum join the inside surface of this region and should be left intact and attached to the body of the urchin and to the periproctal region. Do not remove it or destroy its connections with the rest of the viscera. Of course, after you remove the surrounding test, it will no longer be supported and will fall onto the viscera below. That's okay. Organ systems are discussed in the order they are revealed by dissection.
Reproductive System
The large space revealed by removal of the test is the perivisceral coelom but other divisions of the coelomic space are present as well (Fig 3, 28-36). The most obvious organs in the perivisceral coelom are the five gonads (Fig 28-36). The gonads are on interambulacral axes and are eitherovaries or testes. In Strongylocentrotus both are orangish but the sperm inside a testis are white whereas ova are orange or brown. (In Arbacia ovaries and eggs are raspberry red. Testes are yellowish with white sperm.) The gonads are arranged around the periphery of the aboral part of the coelom and each empties to the exterior via a gonoduct running to a gonopore on a genital plate. Carefully remove the gonads without disturbing any other tissues, especially the gut lying near them. Urchins are gonochoric, fertilization is external, development is indirect, and the larva is a bilaterally symmetrical planktotrophic echinopluteus (Fig 28-44)
Digestive System
Find the large, complicated, white, calcareous mass in the center of the floor of the body cavity. This is Aristotle's lantern, the device that supports and operates the five teeth in the mouth (Fig 3, 28-38). Notice its many calcareous ossicles, their muscles, and its pentamerous symmetry. The lantern is enclosed in its own peripharyngeal coelom surrounded by the thin transparent peripharyngeal peritoneum (Fig 4). Avoid damaging the peritoneum or the nearby muscles.
The mouth opens into a spacious pharynx entirely within the lantern (Fig 28-36). The pharynx rises vertically through the core of the lantern and emerges from it as the esophagus (Fig 3, 28-36). The axial complex, which includes the axial hemal vessel (= axial gland) and heart, axial canal (= axial sinus), and stone canal, also emerges from the center of the lantern but is smaller in diameter and extends to the madreporite at the aboral pole (Fig 3, 4, 28-36).
Shortly after exiting the lantern, the esophagus turns toward the periphery and, upon reaching the body wall, widens dramatically to become the stomach (Fig 3, 28-36). The stomach makes a complete counterclockwise circle around the inside of the test on the floor of the perivisceral coelom.
Figure 3. Aboral view of Strongylocentrotus drobachiensis with the aboral test removed. A portion of the intestine has also been removed to reveal the underlying stomach. The rectum and axial complex are shown attached to the periproct, which is upside down and unsupported. Arrows show the path taken by food moving through the gut. Echinoid11La.gif
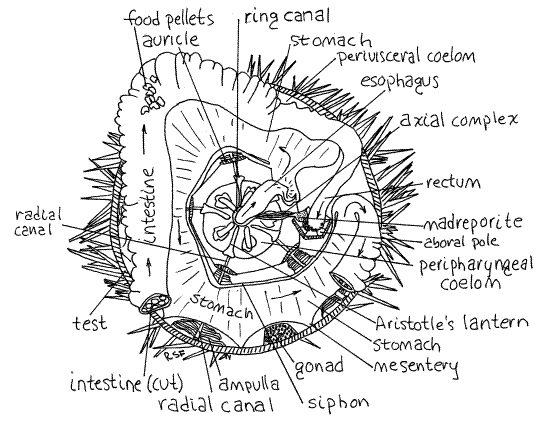
The siphon is a thin tube on the inside curve of the stomach (Fig 3, 28-36). Its lumen connects with that of the stomach at the beginning and end of the stomach. It is a water channel bypassing the stomach, presumably to route water around the digestive and absorptive region of the gut and thereby avoids diluting digestive enzymes with water ingested with the food. A similar device is present in echiurans, some limpets, and some polychaetes (i.e. capitellids). Hemichordates use gill slits to eliminate excess water from the gut and sipunculans have a ciliated channel that may perform the same function. The siphon, which is easily seen, diverges from the beginning of the stomach and rejoins it at the junction with the intestine. It thus makes a convenient marker for recognizing the beginning and end of the stomach. (The siphon of S. purpuratus is not so obvious.)
At the end of the stomach the gut reverses direction and, now known as the intestine, makes a second circle, this one clockwise and aboral to the stomach (Fig 3). The intestine is wide and flat like the stomach but paler in color. It is attached by mesenteries relatively high on the walls of the test and consequently is often damaged during removal of the aboral hemisphere of the test. The stomach is also attached by mesenteries but lower on the test so it is less likely to be damaged. The stomach and intestine are very delicate and easily torn. They are difficult to study if they have been damaged so do your best to keep them intact. The intestine is often filled with irregular, green or brown food pellets. Make a small opening in the intestine to reveal the pellets. Starved specimens will have no pellets.
Upon completing its clockwise loop the intestine becomes the rectum which extends vertically (aborally) to the anus in the periproct (Fig 3, 28-36). Hopefully, the rectum is still attached to the periproct. Find the anus on the outer surface of the periproct and demonstrate its continuity with the rectum by slipping a needle or probe into it and watching it appear in the rectum.
Water Vascular System
" Cut the esophagus where it joins the stomach and then cut the rectum. Sever the mesenteries attaching the stomach and intestine to the test and carefully remove the gut, leaving only the esophagus and rectum.
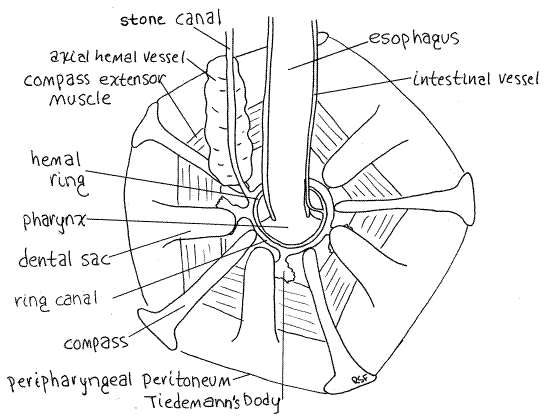
The water vascular system is one of the many coelomic compartments for which echinoderms are famous. It is filled with slightly modified seawater which presumably enters via the pores of the madreporite. Its central feature is the water ring, or ring canal (= water ring), located inside Aristotle's lantern. This small, transparent, inconspicuous ring circles the esophagus as it (the esophagus) emerges from the lantern (Fig 4). The ring canal lies against the inner wall of the lantern and is not in contact with the esophagus. To locate it find the opening in the center of the top of the lantern from which the esophagus emerges. A short distance inside (orally) this opening is a mesentery extending across the opening to the wall of the esophagus. This mesentery separates the lantern coelom (= peripharyngeal coelom) from the perivisceral coelom. The ring canal is located on this mesentery touching the ossicles of the lantern but not touching the esophagus. Actually, there are two similar membranous rings here (Fig 4). The larger, slightly oral ring is the ring canal. The other lies on top of it and is slightly smaller in diameter. It is the hemal ring and is a blood vessel belonging to the hemal system.
The stone canal is a small, membranous, transparent tube that emerges from one side of the water ring and extends aborally to the madreporite (Figs 3, 4, 28-36). The canal itself is a small diameter tube but is enveloped by a large brown axial hemal vessel that is easier to find.
Five small Tiedemann's bodies are regularly spaced around the periphery of the ring canal (Fig 4, 28-36). In Strongylocentrotus they are flat, rectangular or bushy sacs adhering to the inner walls of the lantern and connected by short ducts to the ring canal. They are inconspicuous and look like dark smudges of pigment on the lantern. Tiedemann's bodies are diverticula of the ring canal in which foreign particles are removed from the water vascular system by phagocytosis. They are interambulacral in position. Polian vesicles are not present.
Each of five radial canals exits the ring canal and makes its way to an ambulacrum on the test (Fig 3, 28-36). The radial canals are hidden from view for much of their length. They exit the oral side of the ring canal, disappear into the lantern, emerge on its outside surface, and pass down the outside of the lantern to the floor of the body cavity. Each passes through the arch of an auricle, and then along an ambulacral axis up the curved wall of the test to the aboral pole. Upon reaching the pole, it emerges through a tentacular pore in an ocular ossicle and ends as a tentacle. The canals are easiest to see on the sides of the lantern where they are slender, slightly raised tubes. They are ambulacral in position. If you tilt the test and look at the side of the lantern, the canals can be seen without magnification.
Figure 4. The aboral surface of Aristotle’s lantern showing the central features of the water vascular system of Strongylocentrotus. Echinoid12La.gif
Look at a radial canal on the inner wall of the test using magnification (Fig 3). Innumerable closely spaced lateral canals arise from it. Each lateral canal leads to an ampulla but these are not bulblike and do not resemble the ampullae of sea stars such as Asterias. Instead they are long flat vesicles standing erect like the pages of a book (Fig 2). Use your tiny needle to separate adjacent ampullae and note their shape.
The tube feet are the respiratory organs of the perivisceral coelom and the ampullae are adapted to maximize gas exchange between the water vascular system and the perivisceral coelom (Fig 28-37A).
With magnification look at the long narrow edge of an ampulla where it touches the test. Somewhere along this edge arise two tiny podial ducts that pass through two podial pores in the test to connect to one tube foot on the outside.
Find an ampulla close to the broken edge of the test and slip a tiny needle into one of its pores. You may have to experiment with the angle of the needle to get it to enter the pore. It will emerge outside the test in the lumen of a tube foot. Remember which foot. Now remove the needle and slip it into the other pore associated with the ampulla. The needle will emerge in the lumen of the same tube foot.
Hemal System
The well-developed hemal system includes a heart, axial hemal vessel, hemal ring canal, and radial hemal canals but the blood is colorless and the system is difficult to observe. Its central feature is the hemal ring adjacent to the ring canal on the inside circumference of Aristotle’s lantern (Fig 4). From the hemal ring arise two intestinal vessels (one to each side of the gut), the axial hemal vessel, five short vessels to the five Tiedemann's bodies, and five radial hemal canals to the ambulacra.
The intestinal canals can be seen arising on opposite sides of the ring and running along opposite margins of the esophagus (Fig 4). The two intestinal vessels are connected with each other by many smaller vessels. The axial hemal vessel can be seen as a soft bulging mass adhering to the stone canal (Fig 4, 28-36). (The axial hemal vessel of Arbacia is bean-shaped).
A small heart is located at the aboral end of the axial hemal vessel immediately below the madreporite (Fig 28-15). It is enclosed in a contractile pericardial sac and its lumen is continuous with that of the axial hemal vessel. Contractions of the pericardium/heart are visible in careful preparations of living animals. The contraction rate is about five beats/minute. The axial hemal vessel is enclosed in a coelomic space, the axial canal (= axial sinus). The axial hemal vessel and axial canal together are sometimes called the axial complex. The stone canal, which is itself a coelomic space, lies beside it. The lumina of the axial canal and the stone canal are continuous below the madreporite (Fig 28-15).
The radial hemal canals exit the hemal ring and pass oral to the lantern and accompany the radial (water) canals through the arches of the auricles to extend along the ambulacral axes to the aboral pole. They will not be seen. No gastric hemal tufts are found in echinoids.
Nervous System
The nervous system is difficult to study in urchins. Five radial nerves arise from the circumoral nerve ring at the base of the lantern and pass along the ambulacral axes between the radial canals and the test (inside the test, Fig 28-36). The nerve ring is not visible in this dissection. Theradial nerves can sometimes be seen by looking at the broken edge of the test. The nerve is wider than the radial canal, but is very thin, like a ribbon. Each nerve passes through an auricle to the nerve ring.
Excretory System
There has been no demonstration of a conventional excretory system in any echinoderm.
Aristotle's Lantern
" Aristotle’s lantern is a complex of 40 skeletal ossicles and 60 muscles that support and operate five teeth. In regular urchins, such as Strongylocentrotus, the teeth are used for scraping algae and other organisms from rocks. Remove the remainder of the gut, axial complex, and madreporite to expose Aristotle’s lantern in your specimen or use a dried preparation saved by the teaching staff from an earlier dissection, or use both.
The lantern occupies its own coelomic compartment, the peripharyngeal coelom, which is enclosed by the peripharyngeal peritoneum (Fig 3, 4). The peristomial gills, which you saw externally on the peristomial membrane, are the respiratory organs of this coelom and supply the numerous, highly active lantern muscles with oxygen.
The lantern is composed of five jaws, each composed in turn of eight ossicles, the largest of which is the pyramid (Fig 28-38). The ossicles are held in place by muscles and connective tissue. The tooth bands are recognizable internally as five flexible, curved, vertical rods emerging from the aboral end of the pyramids on the interambulacral axes. The peripharyngeal mesentery forms a bulging membranous dental sac around the growing aboral end of each tooth. The oral end of the tooth band is exceedingly hard and protrudes from the mouth as a tooth (Fig 28-30A). The teeth are worn away at the hard oral end but new tooth material is secreted continually at the soft aboral end in the dental sac.
References
Brooks WK . 1890. Handbook of Invertebrate Zoology. Bradlee Whidden, Boston. 352p.
Brown FA . (ed) 1950. Selected Invertebrate Types. Wiley, New York. 597p.
Bullough WS . 1958. Practical Invertebrate Anatomy (2 nd ed). MacMillan, London. 483p.
Cavey MJ, Märkel K . 1994. Echinoidea, pp. 345-400 in Harrison FW, Chia F-S. (eds). Microscopic anatomy of invertebrates, vol 14. Echinodermata. Wiley-Liss, New York. 510pp.
Coe WR. 1912. The echinoderms of Connecticut. Bull. Conn. State Geo. Nat. Hist. Surv. 19:1-152.
Ho J-S . 1978. Laboratory Manual for Invertebrate Zoology Emphasizing Marine Forms. Hwong, Los Alamitos, Calif. 152p.
Hyman LH . 1955. The Invertebrates: Echinodermata, vol. IV. McGraw-Hill, New York. 763pp.
Lawrence J. 1987 . A Functional Biology of Echinoderms. Johns Hopkins, Baltimore. 340p.
Moore RC. (ed). 1966. Treatise on Invertebrate Paleontology, Part U Echinodermata, Asterozoa-Echinozoa, vols 1 and 2. Geological Society of America and University of Kansas Press, Boulder.
Reid WM . 1950. Arbacia punctulata, pp. 528-538 in Brown FA. (ed) Selected Invertebrate Types. Wiley, New York. 597p.
Ruppert EE, Fox RS, Barnes RB. 2004. Invertebrate Zoology, A functional evolutionary approach, 7 th ed. Brooks Cole Thomson, Belmont CA. 963 pp.
Smiley S. 1994. Holothuroidea, pp. 401-471 in Harrison FW, Chia FS (eds.). Microscopic Anatomy of Invertebrates vol. 14 Echinodermata. Wiley-Liss, New York. 510p.
Supplies
Dissecting microscope
Compound microscope
Slides and coverslips
bleach
Seawater
Isotonic magnesium chloride
Dissecting set with microdissecting tools
Cleaned, bleached test, with periproct ossicles intact
Living or preserved regular urchin