Invertebrate Anatomy OnLine
Phascolopsis gouldi ©
Peanut worm
4jul2006
Copyright 2003 by
Richard Fox
Lander University
Preface
This is one of many exercises available from Invertebrate Anatomy OnLine , an Internet laboratory manual for courses in Invertebrate Zoology. Additional exercises, a glossary, and chapters on supplies and laboratory techniques are also available at this site. Terminology and phylogeny used in these exercises correspond to usage in the Invertebrate Zoology textbook by Ruppert, Fox, and Barnes (2004). Hyphenated figure callouts refer to figures in the textbook. Callouts that are not hyphenated refer to figures embedded in the exercise. The glossary includes terms from this textbook as well as the laboratory exercises.
Systematics
SipunculaP, Sipunculidea C, Sipunculidae F (Fig 14-14)
Sipuncula P
Sipuncula is a small taxon of 150 described species of marine benthic animals known as peanut worms or star worms. Most are infaunal and wedge themselves into crevices, burrow into soft sediments, or bore into calcareous substrata. Sipunculans are relatively large worms, most being about 10 cm in length but with a range extending from 2 mm to 70 cm.
The anterior end of the body is an eversible introvert with the mouth at its tip. A ring of hollow, ciliated tentacles surrounds the mouth and is used for feeding and gas exchange. The coelom is divided into anterior and posterior compartments. There is no hemal system and the coelom is the fluid transport system. The respiratory pigment, hemerythrin, is present in coelomocytes. The gut is coiled on itself and J-shaped, with a dorsal anterior anus. Two large metanephridia are present. The sexes are separate with gametes maturing in the coelom for later release to the sea through the metanephridia. Cleavage is spiral with a molluscan cross. Development may be direct or with either a trochophore or pelagosphera larva.
Sipunculidea C
The tentacles of sipunculideans form a circle around the mouth whereas those of the sister taxon, Phascolosomatidea C, are dorsal to the mouth. Most are burrowers but some inhabit empty snail shells.
Laboratory Specimens
Sipuncula is an homogeneous taxon of similar species. This exercise is written for Phascolopsis gouldi (Sipunculidae), which occurs from Connecticut to Florida on the American east coast but other species can also be used. Phascolopsis may reach almost 30 cm in length but most are smaller. Phascolopsis constructs burrows in muddy sand in shallow coastal waters. Themiste alutacea (Golfingidae) is a small peanut-shaped species that bores into calcareous substrata or inhabits interstices in mussel beds. Sipunculus nudus (Sipunculidae) is a large sand-burrowing species.
Relaxed living or preserved specimens can be used for this study and, because of the morphological uniformity of sipunculans, other species can be substituted for Phascolopsis if convenient. Biological supply companies may not be careful about the identity of the preserved specimens they ship and you may not know the identity of your specimen. Parenthetical notes are used to indicate differences between Phascolopsis and other genera.
Specimens should be studied in a long narrow dissecting pan with a dissecting microscope. (Themiste can be dissected in an anchovy tin.) If your specimen is preserved, it should be immersed in tapwater. If it is living, it should be in isotonic magnesium chloride or 5-10% ethanol (non-denatured) in seawater (See Techniques and Supply chapters). Magnesium chloride is the better anesthetic but requires about 24 hours immersion to be effective. Ethanol will anesthetize the worm in about 30 minutes but muscles will remain partly contracted. Some active, unrelaxed worms in seawater should be available for behavioral observation.
If you immerse an active worm in ethanol, watch it closely. It will probably extend and retract its introvert at least once as it succumbs to the anesthetic. Observe this event if it occurs.
External Anatomy
1. Phascolopsis is long, slender, cylindrical, and wormlike, moreso than most sipunculans (Fig 1). (Themiste alutacea is short and pear- or peanut-shaped, Fig 14-9). The body is divided into an anterior, fully retractable introvert and a posterior trunk (Fig 14-8C). The introvert is about ¼ the length of the body and is smaller in diameter than the trunk. (Sipunculus nudus has a very short introvert and a long cylindrical body, Fig 14-8A). The introvert is retracted into the anterior trunk by turning it outside in.
Find the anus on the mid-dorsal line between the trunk and introvert (Fig 1, 14-9). It is a small opening on a low protuberance. You now have landmarks for finding anterior and dorsaland you can determine posterior, ventral, right, and left.
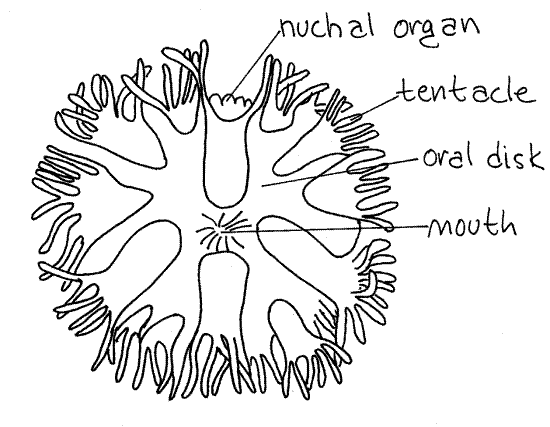
The extreme anterior end of the introvert is the flattened oral disc with the mouth at its center (Fig 2). The mouth and oral disc are surrounded by a ring of up to 200 small, unbranchedtentacles used for feeding and gas exchange (Fig 2, 14-8C). (The number and shape of the tentacles varies with species. Those of Themiste are branched, Fig 14-10C). Note that the interior of each tentacle is pink (in life). The color is due to the presence of the respiratory pigment hemerythrin in vessel-like coelomic channels in the tentacles. There is a longitudinal ciliated groove on the frontal surface of each tentacle.
A small, chemosensory nuchal organ (nuchal = neck) is located on the dorsal surface of the introvert and overhangs the dorsal margin of the oral disc. It bears three longitudinal ciliated grooves. (The tentacles of Phascolosoma form a partial ring around the nuchal organ and do not encircle the mouth.)
The oral disc, mouth, and tentacles cannot be seen if the introvert is even slightly retracted. If the oral disc is not exposed, it will not be visible until the body cavity has been opened.
Figure 1. View of Phascolopsis gouldi from the left. Redrawn from Stephen and Edmunds (1972). Sipunc23L.gif
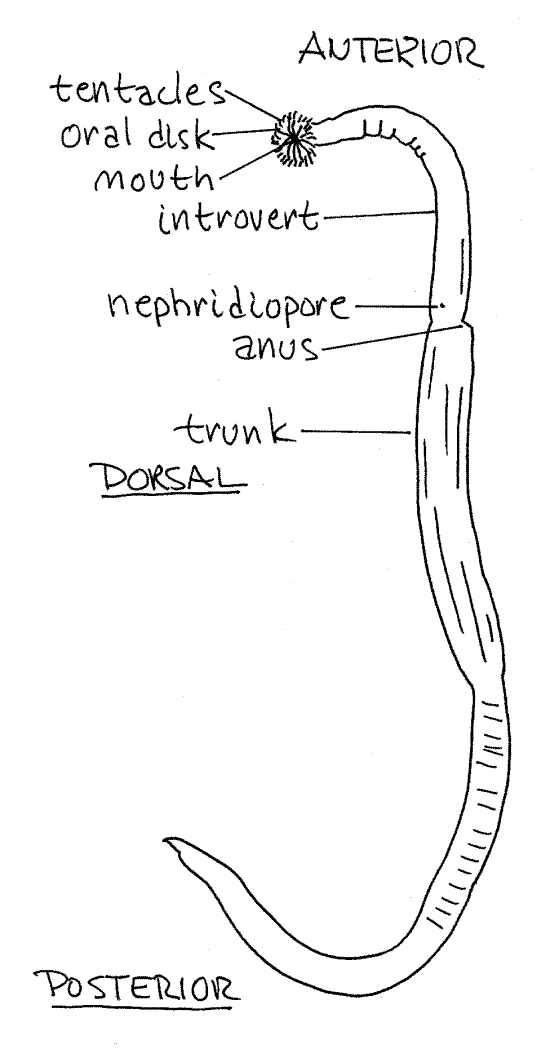
The surface of the worm is covered by a thick cuticle and bands of longitudinal body wall muscles can be seen through the integument of the trunk. (Themiste, Golfingia, andPhascolosoma may have cuticular hooks on the introvert. Soft papillae or scales may also be present. Phascolopsis agassizi has especially well-developed papillae and which give it a fuzzy appearance.)
Two small nephridiopores are located just anterior and ventrolateral to the anus (Fig 1). Sometimes the nephridiopores are on small papillae and easy to find but often the papillae are flattened and the openings are not detectable externally. (The location of the nephridiopores varies with species and they are sometimes posterior to the anus as in Themiste zostericola.)
Figure 2. En face view of the oral disc and mouth of Phascolopsis. Redrawn from Andrews (1890). Sipunc24L.gif
Internal Anatomy
" With fine scissors, cut about 3 mm from the posterior tip of the body. Coelomic fluid will flow from this opening. Coelomic fluid forms clots when outside the coelom.
>1a. If you have a living specimen, place a drop of coelomic fluid on a slide and note its distinctly pink color (in living specimens). Mix with a drop of seawater, add a coverslip, and use the compound microscope to search for cells. By far the most common are small, transparent, flat, spherical, nucleated hemerythrocytes which contain the pink respiratory pigment, hemerythrin. (The pink color is not apparent in the transmitted light of the compound microscope.) The coelomic fluid also contains amoebocytes of various sizes.
Sipunculan gametes mature in the coelom and you may see some of them. Developing ova are conspicuous, heavy-walled spheres of various sizes. The large nucleus is sometimes visible in the center of these cells. If ova are present, the coelomic fluid will appear distinctly grainy, even to the unaided eye.
Sperm are tiny, thimble-shaped cells, each with a long flagellum. Coelomic sperm are typically immobile with inactive flagella. They are activated by contact with seawater and the seawater you used to make the wetmount may have accomplished this. <
" Place the worm in the center of the dissecting pan with the ventral side down, against the wax of the pan. The anus, which is dorsal, should be facing up, toward you. Insert one point of your finest scissors in the posterior opening made earlier and cut anteriorly, all the way to the anterior end of the introvert, to open the body cavity. Make this and subsequent incisions using magnification and be careful that you do not cut any of the organs in the coelom. Try to keep the incision on the dorsal midline using the white nerve cord (Fig 3) on the ventral midline as a landmark. Stay as far from the nerve cord as you can. At the base of the introvert, swerve a little to one side to avoid the anus. Pin the animal to the wax of the dissecting pan as you cut, being sure to plan ahead so there will be room in the pan for the extended introvert. Insert the pins at 45 ° angles to the wax.
" If the introvert is still retracted, and hence outside in, open it anyway. Do this by inserting the tip of your fine scissors in its open (anterior) end and cut the doubled body wall posteriorly to the concealed oral disc and stop cutting when you reach the tentacles. Grasp the introvert with forceps and pull it anteriorly to evert it. Pin it in the everted position.
The gut and nephridia may have become entangled with each other or with the retractor muscles. If this is the case, attempt to extricate them so they hang free in the coelom.
Body Wall
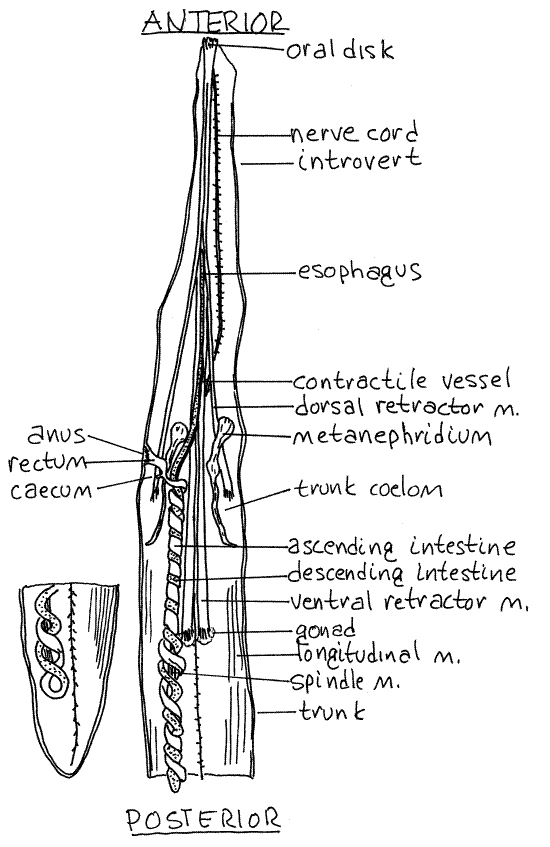
The space you opened is the posteriormost of two coelomic compartments and is the trunk coelom, or perivisceral coelom (Fig 3, 14-10A). The body wall as revealed by your incision consists of a thick external cuticle, thin epidermis, thin connective tissue layer, outer circular and inner longitudinal muscles, and the peritoneum (Fig 14-10B). The epidermal and connective tissue layers are thin, inconspicuous, and are not apparent in gross dissection. The epidermis is a monolayered epithelium that secretes the cuticle and is ciliated only on the tentacles and oral disc. It contains numerous multicellular, secretory epidermal glands which appear as clear microscopic ovals in the cuticle. They are easily seen on the inside surface of the wall of the introvert. The cuticle of some species is locally sclerotized to form hardened hooks or tubercles.
The longitudinal muscle layer of Phascolopsis is very thick in the trunk where it is divided by deep grooves into 30-40 longitudinal bundles (Fig 3). The longitudinal musculature of the introvert is thinner and is not divided into bundles. (The muscles of many sipunculans, including Golfingia and Themiste, are not in bundles but form a continuous sheet in the body wall. InSipunculus both the circular and longitudinal muscles are in bundles.)
Examine the longitudinal muscles with high power of the dissecting microscope. The bundles and the grooves between them are overlain by the thin peritoneum, which is a monolayered, ciliated, squamous epithelium. The circular muscles form a thinner layer than the longitudinals and can be seen just inside the cuticle and outside the longitudinals.
Figure 3. Dorsal view of the trunk coelom of Phascolopsis. Sipunc25L.gif
Coelom
The small anterior tentacular coelom is confined to the oral disc whereas the posterior trunk coelom is much larger and is the major body cavity. The two coeloms are separated by a complete septum in the oral disc. Both are lined by a peritoneum on which tufts of cilia occur on isolated multiciliated cells. Both coeloms contain hemerythrocytes.
Tentacular Coelom
The tentacular coelom is tiny and most of it is difficult to see in gross dissection. (In living specimens its hemerythrin gives it a dark pink color and makes it easier to see.) The central feature of the tentacular coelom is a ring canal around the pharynx at the base of the oral disc. The ring canal may, at best, be faintly visible through the body wall at the base of the tentacles. Ciliated tentacular canals arise from the ring and three such canals extend into each tentacle. Two lateral afferent tentacular canals deliver coelomic fluid to the tentacle and a single central efferent tentacular canal drains it back to the ring. The tentacular canals are responsible for the faint pink color of the tentacles of living animals.
Figure 4. Cross section of the esophagus and contractile vessel of Phascolopsis. Redrawn from Cuénot (1900). sipunc26L.gif
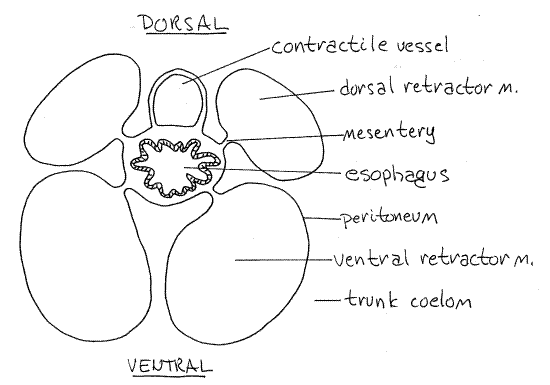
The most obvious part of the tentacular coelom is the contractile vessel, or compensation sac, Fig 3, 14-10A,B). This long, pink or brown diverticulum extends far posteriorly from the tentacular ring into the trunk coelom. It is an evagination of the tentacular coelom into the trunk coelom. It lies on and is attached to the dorsal surface of the esophagus (Fig 4, 14-10). (The contractile vessel is small and inconspicuous in Phascolosoma agassizii and is reduced in Golfingia. Sipunculus has two contractile vessels. In some sipunculans, notably Themiste, the contractile vessel has several long, narrow, blood vessel-like branches extending into the trunk coelom.)
The wall of the contractile vessel is muscular and its peritoneum is ciliated. The muscles cause pulsations of the sac and the cilia generate a bidirectional current to move hemerythrocytes. The contractile vessel is a transport system for exchanging materials between the two coeloms. Oxygen, for example, is transported from the tentacles to the trunk by these vessels. Nutrients from the gut are moved in the opposite direction.
Use high power of the dissecting microscope to examine the contractile vessel for evidence of ciliary currents and, if your specimen is not relaxed, for muscular pulsations.
Trunk Coelom
The trunk coelom is very large and extends uninterrupted from the oral disc through the introvert and trunk (Fig 3, 14-10A). It is the coelom of the trunk and most of the introvert. It is not partitioned by septa or mesenteries although small mesenteries are present. The contractile vessel extends far posteriorly into the trunk coelom.
Retractor Muscles
Two pairs of large introvert retractor muscles extend from the body wall of the trunk to the esophagus at the anterior end of the introvert. Their contractions pull the introvert into the trunk. It is re-extended by the action of the body wall muscles on the coelomic fluid.
The dorsal introvert retractor muscles originate on the dorsal body wall whereas the ventral introvert retractor muscles arise on the ventral wall (Figs 3, 4, 14-10A,B). The ventral muscles are longer than the dorsal and originate farther posteriorly. The retractor muscles are long, flat, white, strap-like muscles that are extensions of the longitudinal muscles of the body wall. (Themiste has only two retractor muscles; the dorsal pair is absent. There are four retractors in Sipunculus but all are short and about the same length).
Use a higher power to examine the posterior end of a dorsal retractor muscle and confirm its continuity with the longitudinal body wall muscles. Anteriorly the four retractor muscles are connected to each other by connective tissue and form a sheath around the anterior gut and contractile vessel (Fig 4).
Digestive System
The sipunculan gut is a long J-shaped loop between the mouth and anus. The two arms of the loop are coiled tightly around each other (Fig 3, 14-10A). Sipunculans are suspension or deposit feeders that collect and ingest small organic particles.
The mouth in the center of the oral disc opens into a short, wide pharynx in the anterior introvert. Posteriorly the pharynx constricts to become the long, narrow, straight esophagus that extends the length of the introvert to the anterior trunk (Fig 3). The dark contractile vessel adheres to the dorsal surface of the pharynx and esophagus (Fig 10-14B). Each retractor muscle is attached to the esophagus by a mesentery (Fig 4). Contractions of the muscles pull the esophagus posteriorly and that in turn pulls the oral disc and introvert back into the trunk coelom.
In the anterior trunk the esophagus initiates the first of many tight coils of the posterior gut and then becomes the stomach. This short segment of the gut is slightly wider than adjacent regions. It begins at the end of the contractile vessel and extends for a little more than one complete coil of the gut.
In its wall can be seen four distinct, pink, longitudinal stripes separated by thin pale stripes. These four stripes are the outer edges of four longitudinal ridges of gelatinous connective tissue that protrude into, and nearly fill, the lumen of the stomach. In living specimens the stomach is easy to recognize due to this striped pink color.
The exceedingly long, coiled intestine exits the stomach and extends far posterior in the trunk as the descending intestine, then reverses direction and climbs back into the anterior trunk as the ascending intestine (Fig 3). In some specimens it may be filled with sand and distended. The mucosal epithelium of the descending coil is secretory and releases hydrolytic enzymes into the gut lumen. (In Sipunculus there are two loops of the intestine. The usual long coiled loop is present but in addition there is a shorter "sipunculus loop" in the anterior intestine. It is characteristic of the family, Fig 14-10A).
The ascending intestine is wider than the descending and lacks its distinct longitudinal stripes. The wall of the ascending intestine bears a ciliated groove in its lumen. The ciliated groove can be seen through the wall of the ascending intestine as a narrow white stripe on the inner, or lesser, curvature of the intestine. The ciliary current in this groove is toward the anus.
The intestine makes its 35-40 coils around a long, slender spindle muscle (Fig 3). This muscle originates anteriorly on the body wall, supports the intestine, and shortens the intestinal loop when it contracts. It inserts on the posterior bend of the intestine and is attached to the ascending intestine by a mesentery. The intestinal loop of most species, including Phascolopsis, hangs freely in the coelom and is attached to the body wall only at the anterior end. (In Phascolosoma agassizii it is attached posteriorly to the body wall and anchors the posterior end of the gut. The spindle muscle of some species of Golfingia is weakly developed. In Sipunculus the intestinal coils are attached to the body wall by numerous small mesenteries).
Four long, slender, threadlike, almost invisible fixing muscles extend from the gut to the body wall, one from the posterior pharynx and three from the intestine.
In the anterior trunk cavity the ascending intestine stops coiling and becomes the short straight rectum which joins the body wall on the anterior, dorsal midline at the anus (Fig 3, 14-10A). There is a tiny, bulbous cecum at the point where the intestine becomes the rectum. (The cecum is absent in some species). The rectum is attached to the body wall by a small, wide wing muscle. The spindle muscle attaches to the body wall with the wing muscle. (In Sipunculus a pair of racemose glands of unknown function form tufts beside the rectum.)
The faint yellowish color of the intestine is due to the presence of a thin layer of chlorogogen cells in the peritoneum. The peritoneum covering the ascending intestine bears numerous ciliated pits called fixed urns but they are not apparent in gross dissection. Chlorogogen cells associated with the urns accumulate foreign particles from the coelomic fluid.
>1b. The mucosal epithelium of most of the gut is ciliated. Place small particles such as carmine, carbon, or eggs or hemerythrocytes from the coelom on the ciliated tentacles or oral disc. Tentacular cilia may move the particles into the pharynx and their subsequent progress along the gut tube can be traced through its translucent walls. <
Respiratory and Fluid Transport Systems
There is no hemal system in sipunculans. The tentacles of the oral disc are probably the primary respiratory surface for crevice dwellers but the general body surface is also important in sand-dwelling species. The tentacular and trunk coeloms are the fluid transport system for the distribution of oxygen. Hemerythrin in the tentacular coelom is oxygenated in the tentacles and the hemerythrocytes moved posteriorly by the ciliated and muscular peritoneum of the contractile vessel. In the trunk coelom oxygen is transferred from hemerythrocytes of the contractile vessel to hemerythrocytes of the trunk coelom for distribution to the tissues. (In Sipunculus coelomic dermal canals extend from the trunk coelom into the integument where they run between muscle bundles. Coelomic fluid circulates through them and they are thought to function in gas exchange and distribution.)
Excretory System
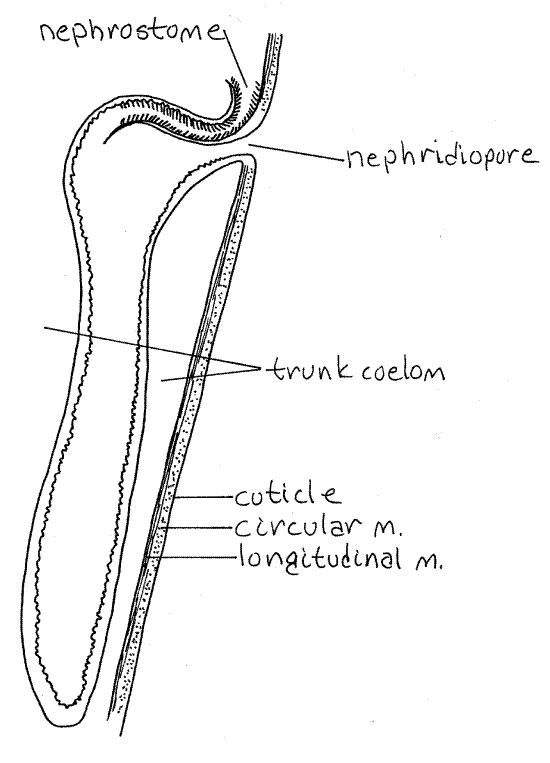
The two large, dark brown metanephridia are long, narrow sacs that hang freely in the coelom (Fig 3, 14-10A). They are attached to the anterior lateral body wall and open to the exterior via the nephridiopores just anterior to the anus. If you could not find the nephridiopores earlier, unpin this region of the worm and find them now.
The nephrostome is a ciliated opening from the trunk coelom into the nephridium (Fig 5). It is located on the dorsal side of the nephridium at the point where the nephridium attaches to the body wall and is covered by a small, transparent, semicircular lip. Most of the nephridium is a long, contractile, blind sac in which mature gametes are stored. The nephridia function as gonoducts through which the gametes exit the coelom. Podocytes are present on the contractile vessel and the metanephridium but their role in excretion is not understood.
Ammonia is probably lost by diffusion across the general body surface and from the tentacles. The nephridia may play a role in osmoregulation.
>1c. The nephrostome opens into a channel in the dorsal nephridial wall that leads into the lumen. Insert a nadel carefully into the nephrostome and trace the channel to its internal opening. Place some carmine/seawater on the peritoneum in front of the nephrostome and watch what happens to it. Find the small puckered internal opening into the nephridiopore on the ventral anterior wall of the nephridium, ventral to the nephrostome. <
Figure 5. Longitudinal section of a sipunculan metanephridium. Sipunc27L.gif
Nervous System
The subepidermal nervous system consists of a brain, nerve ring, and ventral nerve cord. The conspicuous, white ventral nerve cord can be seen on the ventral midline of the trunk coelom (Fig 3, 14-10A). (Hemerythrin may render the nerve cord of Sipunculus nudus pink.) Look for the unpaired lateral nerves that exit it at irregular intervals to enter the body wall where they innervate its muscles. Use high power to look for the nerves. These nerves are evident along the entire length of the nerve cord and can be seen best by lifting the cord slightly with a bentminuten nadel.
There are no ganglionic swellings of the nerve cord and no hint of segmental arrangement of the lateral nerves. The ventral nerve cord extends anteriorly for the length of the coelom and bifurcates ventral to the anterior pharynx to form two circumenteric connectives that enter the wall of the gut and extend dorsolaterally around the pharynx at the base of the tentacles (Fig 14-10A).
The two connectives form a nerve ring around the pharynx. They join dorsally at the brain which lies on the midline in the roof of the pharynx between the insertions of the two dorsal retractor muscles. The brain is partly surrounded by the tentacular coelom which supplies it with oxygen. The nerve ring and brain are imbedded in connective tissue and are difficult to expose but pink coelomic fluid surrounding it may make the brain region easier to locate.
Observe the brain and nerve ring through the surrounding tissues. Both can be seen faintly through the retractor muscle sheath covering the base of the tentacular crown. A pair of tiny, dark pigment cup ocelli, or eyes, embedded in the brain serve as landmarks. A presumably chemosensory nuchal organ is located dorsal to the mouth on the edge of the oral disc (Fig 2, 14-10C).
>1d. Insert the tip of your fine scissors into the mouth and cut posteriorly on the dorsal midline for a few millimeters. Look at the resulting sectioned dorsal wall of the pharynx and you will see the brain in sagittal section. The tentacular ring canal of the tentacular coelom may also be visible in cross section at the anterior ventral edge of the brain. Find the nuchal organ dorsal to and anterior to the brain. Note the large longitudinal folds in the walls of the pharynx. <
Reproductive System
Fertilization is external and, although sipunculans are gonochoric, there is no sexual dimorphism. Fertilization is external. The two retroperitoneal gonads are inconspicuous, frilly, transverse ridges across the base of the two ventral retractor muscles (Fig 3, 14-10A).
Germ cells do not ripen in the gonads nor are gametes stored there so they are never large. Germ cells are released into the trunk coelom where they complete their maturation and become spermatozoa or ova.
Mature gametes accumulate in the metanephridia prior to release. They are then forced out the nephridiopore by contractions of muscles in the nephridial wall. Spiral cleavage produces a trochophore larva in some species, including Phascolopsis.
Behavior
>1e. Watch a living, unrelaxed worm in a finger bowl of seawater as it retracts and extends its introvert. Use magnification to observe the tip of the introvert as it folds in on itself or extends. <
>1f. Place a worm in a bowl of sand and watch it burrow by extending the introvert into the sand. <
References
Andrews EA . 1890. Notes on the anatomy of Sipunculus gouldii Portuales. Stud. Biol. Lab. Johns Hopkins Univ. 4:389-430, pls. 44-47.
Brown FA . 1950. Phascolosoma gouldii, pp 309-317 in Brown FA (ed.) Selected Invertebrate Types. Wiley, New York. 597p.
Cuénot, L. 1900. Le Phascolosoma commun (Phascolosoma vulgare de Blainv.) [in] Boutan, L. (ed.) Zool. descriptive des invertebres, Paris1:386-422.
Cutler EB. 1973. Sipuncula of the western North Atlantic . Bull. Am. Mus. Nat. Hist. 152(3):105-204.
Cutler EB. 1994. The Sipuncula Their systematics, Biology, and Evolution. Cornell Univ. Press, Ithaca. 453 pp.
Fisher WK. 1950. The sipunculid genus Phascolosoma. Ann. Mag. Nat. Hist. ser 12, 3:547-552.
Hyman LH . 1959. The Invertebrates: Smaller coelomate groups, vol. V. McGraw-Hill, New York, 783p.
Kohn AJ, Rice ME. 1971. Biology of Sipuncula and Echiura. Bioscience 21:5833-584.
Metalnikoff S. 1900. Sipunculus nudus. Z. Wiss. Zool. Abt. A 68:261-322.
Peebles F, Fox D. 1933. The structure, functions, and general reactions of Dendrostoma zostericola. Bull. Scripps Inst. Oceanogr. Ser 3:201-224.
Pilger JF. 1982. Ultrastructure of the tentacles of Themiste lageniformis (Sipuncula). Zoomorph. 100:143-156.
Rice ME. 1981. Larvae adrift: Patterns and problems in life histories of sipunculans. Amer. Zool. 21:605-619.
Rice ME, Todororic M , eds. 1970. Proceedings of the International Symposium on the Biology of the Sipuncula and Echiura. Inst. Biol. Res. Yugoslavia and Smithsonian Institution, Washington. Vol. 1 (355p), vol. 2 (254p).
Stephen AC, Edmonds SJ . 1972. The phyla Sipuncula and Echiura. British Mus. Nat. Hist, London. 528 p.
Ruppert EE, Fox RS, Barnes RB. 2004. Invertebrate Zoology, A functional evolutionary approach, 7 th ed. Brooks Cole Thomson, Belmont CA. 963 pp.
Supplies
Compound microscope
Dissecting microscope
Dissecting pan (kippered herring tin or aluminum ice tray for Phascolopsis or
Sipunculus, anchovy tin for Themiste)
Dissecting set with microdissecting tools
Isotonic magnesium chloride
Seawater
Living or preserved sipunculan
#1 stainless steel insect pins
Living sipunculans for behavioral observations