Invertebrate Anatomy OnLine
Ophioderma brevispina ©
Brittlestar
25may2007
Copyright 2001 by
Richard Fox
Lander University
Preface
This is one of many exercises available from Invertebrate Anatomy OnLine , an Internet laboratory manual for courses in Invertebrate Zoology. Additional exercises, a glossary, and chapters on supplies and laboratory techniques are also available at this site. Terminology and phylogeny used in these exercises correspond to usage in the Invertebrate Zoology textbook by Ruppert, Fox, and Barnes (2004). Hyphenated figure callouts refer to figures in the textbook. Callouts that are not hyphenated refer to figures embedded in the exercise. The glossary includes terms from this textbook as well as the laboratory exercises.
Systematics
Echinodermata P, Eleutherozoa, Cryptosyringida, Ophiuroidea C, Ophiurida O, Ophiodermatidae F, (Fig 9-26, 27-12, 28-62)
Echinodermata P
Echinoderms are secondarily radially symmetric deuterostomes whose ancestors were bilaterally symmetric. The adult radial symmetry is pentamerous with body parts occurring in fives or multiples thereof. Echinoderms have strong affinities with the ancestral trimeric deuterostomes especially in the tripartite organization of the coelomic cavities. Echinoderm larvae have the coelom divided into three regions, as is typical of the early coelomates, and these regions have important adult derivatives. All echinoderms are marine and benthic. About 6000 Recent species are known but the fossil record includes 13,000 extinct species.
An important echinoderm apomorphy is the water vascular system that in most groups functions in support of locomotory tube feet but is also important in gas exchange, excretion, and feeding. The body wall includes a thick connective tissue dermis in which calcareous ossicles (little bones) are almost always an important component. These ossicles make up an endoskeleton which assumes different forms in different taxa. In most echinoderms calcareous spines of various sizes and shapes arise from the dermis and extend from the body surface and are alluded to by the name echinoderm (= spiny skin). The connective tissue is mutable and its consistency is under nervous control.
Excretion in echinoderms is accomplished by simple diffusion of metabolic wastes (ammonia) across thin permeable regions of the body wall. A variety of gas exchange structures, including the tube feet, is found in various echinoderms. A hemal system is present but its role in transport is still poorly understood and the chief transport system is the circulating fluid of the various coelomic compartments. The hemal system may be through transport system that delivers nutrients from the gut to these compartments for local distribution. The nervous system consists of two central intraepidermal nerve rings from which arise radial nerves to the periphery. Echinoderms are gonochoric and fertilization is usually external.
Eleutherozoa
Eleutherozoans are mobile echinoderms in which the oral surface is oriented against the substratum. A madreporite and locomotory tube feet are present. Polian vesicles and Tiedemann’s bodies may be present on the ring canal. Movable spines are present. Eleutherozoa includes all Recent echinoderms except for its sister taxon, Crinoidea.
Cryptosyringida
Cryptosyringida includes Ophiuroidea, Echinoidea, Holothuroidea, all with closed ambulacra in which the radial nerve is internalized and protected by ossicles (Fig 28-20).
Ophiuroidea C
Also known as brittle stars or serpent stars, ophiuroids have long sinuous arms sharply demarcated from the central disk. The arms contain vertebra-like ossicles and are very flexible, exhibiting a motion reminiscent of that of snakes (ophio = snake). About 2000 living species are known.
The ophiuroid gut ends blindly, there being no anus or intestine. Digestion and absorption occur in the stomach and pyloric ceca are absent. The digestive system is confined to the central disk with none of it extending into the slender arms. The madreporite is oral and the tube feet lack suckers. The gonads open into invaginations of the integument which also serve as respiratory organs. The larva is the ophiopluteus.
Laboratory Specimens
Ophioderma brevispina is a common brittle star of submerged grass beds on the east coast of the Americas from Cape Cod to Brazil. This exercise applies specifically toOphioderma brevispina, which is a dark, smooth species with short inconspicuous spines. Other genera such as Ophiura can be used but will differ slightly from these descriptions. Preserved specimens from commercial suppliers may belong to any of several genera. As usual, use of living specimens is preferable to preserved and permits behavioral observations. Living specimens should be observed initially in seawater and then transferred to isotonic magnesium chloride. Preserved specimens should be immersed in tapwater.
Behavior
>1a. Place small crustaceans, such as grass shrimp, or even pieces of chopped shrimp, in a 20-cm culture dish with living Ophioderma. Watch a brittle star lasso a shrimp with one of its arms and stuff it into its mouth (Fig 28-27C-E). Ophioderma will eat until the aboral disk surface is bulging and will sometimes rupture its body wall by overeating. <
>1b. Place a living, active Ophioderma in a large dish or aquarium of seawater and watch it move across the surface. It uses two lateral arms as oars and with them rows across the substratum (Fig 28-21A). The leading arm extends ahead to sense environmental conditions in the path and two others trail behind. The role played by each arm changes and depends on the direction of travel. Any arm can play any role. <
>1c. Turn a living brittle star over in a large dish so its oral surface is up and its aboral surface against the substratum. Is the animal rendered helpless by this or can it right itself? Observe the role of the arms in the "righting response". <
>1d. Place a living, unrelaxed Ophioderma in a dish of sand and watch its behavior. Does it burrow into the sand? Observe the sequence in which its parts disappear into the sand. Try to determine how the animal digs. <
External Anatomy
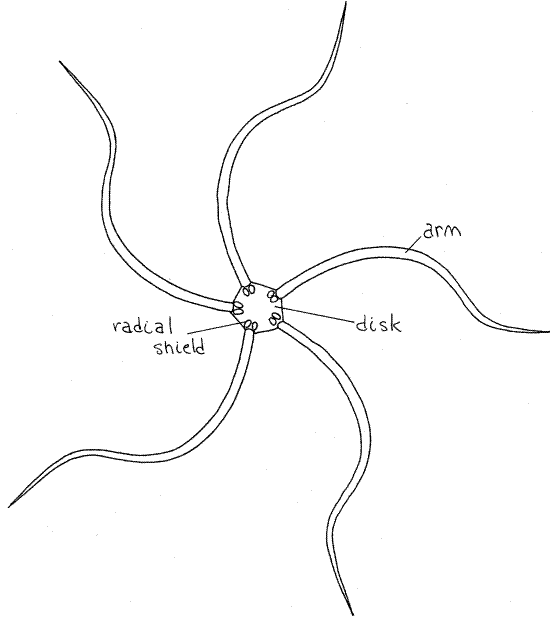
Transfer your specimen from seawater to isotonic magnesium chloride. The dark upper side of Ophioderma is the aboral surface and the pale side opposite it is the oral surface. Note the well defined central disk (Fig 1) from which extend five long snakelike arms (ophio = snake.) The aboral-oral axis runs through the center of the disk perpendicular to the surfaces and is the axis of radial symmetry. Structures remote from the axis are said to be peripheral whereas those near the axis are central. In radially symmetrical animals anterior-posterior, dorsal-ventral, right-left are not applicable.
Imaginary axes radiate outward like spokes from the center of the disk. A radial axis passing from the center of the disk outward along the midline of any arm is a radius, or ambulacral axis, of which there are five. Any axis bisecting the angle between any two adjacent arms is an interradial, or interambulacral axis, and there are five of these also.
Aboral Disk Surface
Look at the aboral surface of the disk and arms with magnification. Ophiuroids have no papulae, no pedicellariae, and no paxillae such as are common in asteroids. The epidermis is a thin, non-ciliated syncytium and most of the body wall is a connective tissue dermis, which contains abundant calcareous ossicles of many sizes and shapes and are often known as shields.
The aboral disk surface of Ophioderma is covered by small, spherical, calcareous dermal granules, which are themselves covered by epidermis (Fig 3). In many genera the aboral disk bears larger, distinct, scalelike ossicles. The granules of Ophioderma are best seen at about 40X magnification. At the base of each arm, but on the disc, are two large oval radial shields(Fig 1, 28-22B). Radial shields are an obvious feature of the aboral disks of most ophiuroids but those of Ophioderma are covered by dermal granules and cannot be seen. There are no distinct muscle layers in the body wall of ophiuroids.
Oral Disk Surface
Turn the animal over and examine the oral surface of the disk with magnification. Unlike that of the aboral disc, the epidermis of the oral disk is ciliated.
>1e. Place a drop of carmine-seawater suspension on the oral disk of a living specimen to test for the presence of cilia. Watch for ciliary currents. Observe a horizontal part of the disk to be sure you don’t mistake gravity currents for those generated by cilia. <
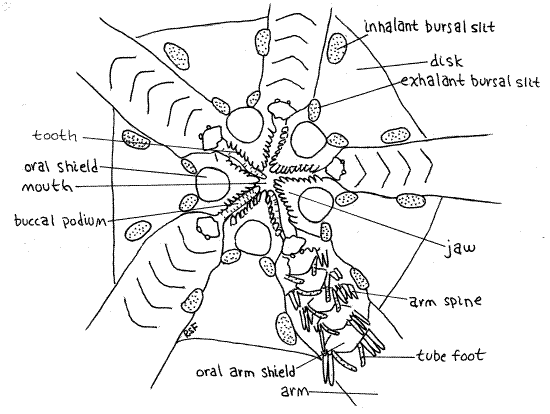
Find the star-shaped m outh at the center of the oral disk (Fig 2, 28-22A). Its margins are formed by five triangular, calcareous jaws, which protrude from the sides into the mouth. Such jaws are characteristic of ophiuroids and bear teeth which, like the jaws, are specialized ossicles, on their inner margins. You can see them clearly by looking into the open mouth. If the mouth is closed, use fine forceps to push two opposing jaws apart. (You cannot do this with preserved specimens.) The mouth opens into a large stomach (Fig 28-24) which occupies most of the space inside the disk, and which can be seen if the mouth is open.
Figure 1. Aboral surface of the brittle star, Ophioderma brevispina. Radial shields are present but not visible on the aboral disk of Ophioderma. Ophio10L.gif
Five large oval ossicles, known as oral disc shields, are located on the surface of the oral disk, one at the base of each jaw (Fig 2). One of them is a tiny bit larger than the others and bears the opening of the madreporite, which you probably will not see. It is a small pore, rather than a sieve. In some genera (Ophiura) this shield bears several obscure pores on its peripheral margin. A microneedle can be inserted into them to confirm their presence.
Several pairs of large, soft buccal tube feet (= buccal podia), are associated with the mouth. They lie in the gap between adjacent jaws. Although obvious in living specimens, they may be retracted and inconspicuous in preserved animals but you should be able to find one or two.
The five pairs of genital bursae are invaginations of the oral surface of the disk (Fig 28-24). In Ophioderma each bursa has two apertures although some species have only one long slit beside the base of the arm (Fig 28-22A). The exhalant bursal slit is closest to the mouth and the inhalant bursal slit is peripheral, farther up the side of the disk (Fig 2). In species with only one slit the peripheral end is inhalant and the central end is exhalant. Use a microneedle to demonstrate the continuity between the two openings of one of the bursae. Slip the needle into one opening and watch for its appearance in the other.
Each bursa is a pouch lined with ciliated epidermis. The bursae are the chief respiratory surface providing oxygen for the organs of the perivisceral coelom and their epidermal cilia generate the ventilating current. The perivisceral coelom surrounds the bursae.
Figure 2. The oral surface of the disk of Ophioderma. Ophio11La.gif
>1f. In living specimens the respiratory current is easily demonstrated with carmine/seawater suspension. The disk must be supported so the bursae are not collapsed by its weight. Use a paper clip to make a cradle to support the disk. Open the paper clip and make a nearly flat triangle of it. Place the paper clip in your dissecting pan and suspend the disk, oral side up, in the center of the triangle, with the arms draped over its wire sides. While watching with 25X magnification, apply some carmine/seawater to the surface of the oral disk and watch for motion of the particles in and out of the apertures. Verify the functions of the inhalant and exhalant bursal slits. <
Arms
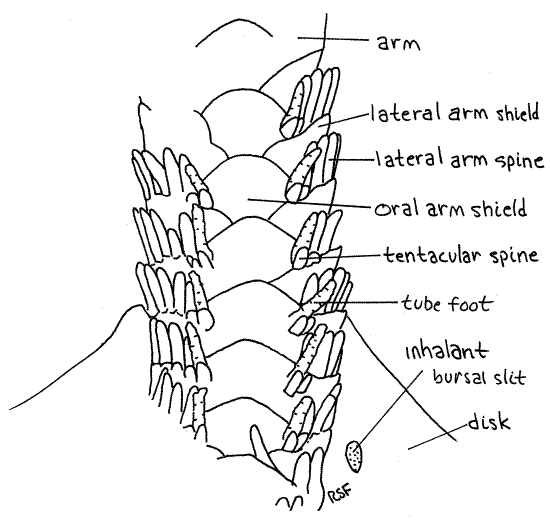
Most of the large dermal ossicles, or shields, covering the aboral surface of the disk are hidden by dermal granules but the shields of the arms are exposed and easily observed. Most important are the four rows of overlapping dermal shields on each arm. A row of aboral arm shields runs along the top of the arm and similar oral arm shields extend along the bottom (Fig 3, 4, 28-23). Each side of the arm bears a row of lateral arm shields. Thus the arm is encircled by a linear series of rings, each consisting of four shields. The name "ophioderma" means snake skin. Is the name apt?
Figure 3. Aboral surface of the arm of Ophioderma brevispina. Ophio12L.gif
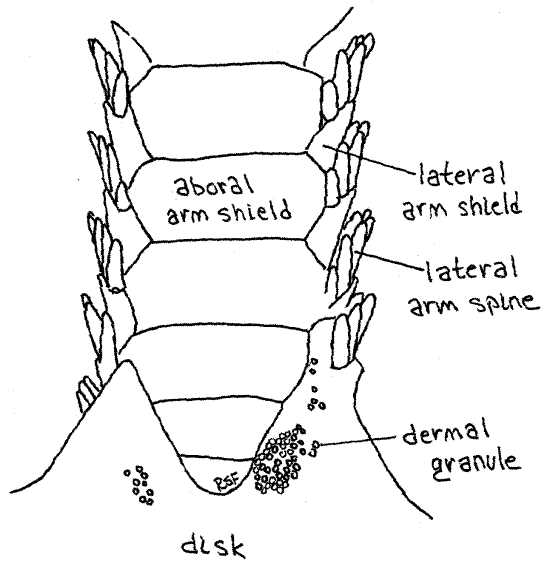
About seven short spines extend from the free margin of each lateral shield and are responsible for the specific name " brevispina" (=short spines). These spines are short inOphioderma brevispinum but much longer in most ophiuroids. A tube foot, or tentacle, arises at the oral end of each row of spines. A pair of small tentacular spines protects the base of eachtube foot, or podium. Ophiuroid tube feet are sometimes called tentacles. They do not have suckers and their ampullae are tiny. Those of preserved Ophioderma are usually retracted but those of some other species may be readily visible. They may resemble arm spines. Touch a suspected tube foot with a microneedle. If it is soft and flexible it is a podium. If hard, then it is a spine.
In some genera, the base of each tube foot is protected by a hinged, movable tentacle scale. Look for it and lift it with a microneedle to reveal the base of the foot.
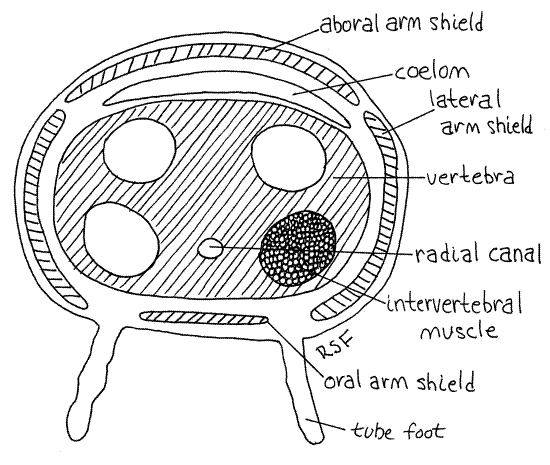
>1g. Pull, rather than cut, the end from an arm so that the arm breaks fairly close to the disk. Look at the broken end with high magnification. Inside is a chain of large, blocky vertebra, or vertebral ossicles (Fig 5, 28-23). These are homologous to the ambulacral ossicles of seastars but they have been internalized and modified to function like vertebrate vertebrae. In effect they are the axial skeleton of the arm. They occupy most of the cross sectional area of the arm. The four arm shields (oral, aboral, and the two lateral shields) can be seen in the walls of the arm surrounding the vertebra. The perivisceral coelom of the disk extends into the arms as a small canal between the vertebra and the aboral arm shields. Four intervertebral muscles on the face of each vertebra attach to the adjacent vertebra and move the two with respect to each other much like the axial musculature of a snake adjusts the relative position of vertebrae and thus the curvature of the spine. A condyle in the center of one end of the vertebra articulates with a socket in the adjacent vertebra and prevents shearing.
The radial canal of the water vascular system runs below the vertebra and above the oral arm shields. You can see the space through which it runs but the canal itself is probably not visible. <
Figure 4. Oral surface of the arm of Ophioderma brevispina. Ophio13La.gif
>1h. Place the detached arm in a dish of household bleach and leave it undisturbed until the vertebrae are exposed as a chain of calcareous blocks. Think about how the four intervertebral muscles could bend the arm left, right, up, or down to account for the characteristic snaky movement of ophiuroid arms. <
Internal Anatomy
" Remove the aboral surface of the disk by lifting it with forceps and cutting around its periphery with fine scissors. Set it aside, in liquid, for the time being. The roof of the stomach is attached to the aboral body wall by numerous tiny connective tissue strands which must be cut. Try to keep the stomach with the oral disk. In preserved specimens the aboral wall of the stomach may adhere tightly to the aboral disk so that it is inevitably lost when the disk is removed. The stomach wall will then be seen as a thin indistinct yellow film on the inner surface of the disk.
The stomach occupies most of the space within the disk and has thin, folded walls that are orangish-brown in living specimens (Fig 28-24). The stomach is a blind pouch surrounded by the perivisceral coelom and no intestine or anus is present. Neither are there pyloric ceca.
>1i. Place a drop of carmine-seawater suspension on the outer surface of the stomach of a living specimen to check for ciliary activity of the perivisceral peritoneum. The carmine particles may be too large for these particular cilia to move. If no ciliary activity is revealed by carmine, try finer particles such as chalk dust from the chalk tray of a blackboard. Make a slurry of chalk dust with seawater and apply it to the surface of the stomach. <
Figure 5. Cross section of the arm of Ophioderma. Ophio14La.gif
Use fine scissors to open the stomach. Look inside and remove any food items it may contain, identifying them if possible. Spread the folded walls apart and note that the stomach bulges outward into the spaces between the bases of the arms to form five peripheral gastric pouches. The inside surface of the stomach wall is densely ciliated. The mouth is the large opening at the center of the oral side of the stomach. It is surrounded by a ring of tiny papillae visible with 25X.
>1j. Place carmine/seawater or wet chalk dust on the exposed inner surface of the stomach of a living specimen and look for evidence of ciliary activity of the stomach epithelium. <
If your specimen is living you may notice that the edges of the folds of the stomach may catch the light and appear to be outlined in iridescent green. This apparent color is due to diffraction by the brush border of microvilli and cilia on the surface of the stomach epithelium and not to pigment.
>1k. Remove a small piece of the stomach wall of a living specimen and make a wetmount with it. Examine it with the compound microscope and look for cilia. <
" Make a radial cut through the floor of the stomach beginning at the mouth. Cut along an ambulacral axis, in line with one of the arms, so that the base of the arm is exposed. Do not cut into the arm. Move the stomach aside so you can see the junction of the arm with the floor of the disk. Note that the base of the arm extends centrally almost to the mouth.
Find the conspicuous vertebral ossicles extending along the length of the arm. They are white, calcareous, and in your view, spindle-shaped, being wide in the middle and tapering at each end. Your view, of course, is of their aboral edge. Find the large, white muscles running between successive vertebrae. The muscles are enclosed in a thin sheath of connective tissue.
Find the genital bursa on each side of the arm. The bursae have very thin, transparent, whitish walls that adhere tightly to the stomach walls. Open a bursa and find the inhalant and exhalant bursal slits from the inside. Slip a minuten nadel into the inhalant aperture of the bursa to see where it appears inside the bursa.
>1l. Put a little carmine/seawater in the open bursa (if your specimen is alive) and see if you can demonstrate a ciliary current. It should be from peripheral to central, or inhalant to exhalant, apertures. <
Locate the piece of aboral body wall you removed earlier from the disk. It consists of a thin epidermis on the outside, a thick, white layer of connective tissue dermis, and a thin innermost peritoneum. The cavity occupied by the stomach is, you recall, the perivisceral coelom and is lined by peritoneum. The ciliated peritoneum covers every surface in the perivisceral coelom, including the body wall.
The gonads are numerous blind, peritoneal pouches that surround each bursa (Fig 28-24). Ripe gonads may be seen bulging into the walls of the bursae. The gonads release gametes into the bursae. The gametes then move into the sea where fertilization occurs.
>1m. If your specimen is living and its gonads are ripe and swollen, open one and make a wetmount of its contents. Examine it with the compound microscope and search for gametes. Eggs are large spheres whereas sperm are tiny and flagellated. <
A few ophiuroids brood embryos in the bursae but Ophioderma does not. Brooding species retain eggs in the bursa. Sperm enter the bursa and fertilization occurs there. Embryos undergo direct development in the bursae and become tiny brittle stars before they are released. Most brittle stars, including Ophioderma, have indirect development with a planktotrophic ophiopluteus larva (Fig 28-28).
It is not practical to study the water vascular, hemal, or nervous systems of Ophioderma in gross dissection.
References
Brooks WK. 1890. Handbook of Invertebrate Zoology. Bradlee Whidden, Boston. 352 p.
Bullough WS. 1958. Practical Invertebrate Anatomy (2 nd ed). MacMillan, London. 483p.
Byrne M. 1994. Ophiuroidea, pp.247-343 in Harrison FW, Chia F-S. (eds). Microscopic anatomy of invertebrates, vol 14. Echinodermata. Wiley-Liss, New York. 510pp.
Reid WM. Ophioderma brevispinum, pp 523-528 in Brown FA. (ed) 1950. Selected Invertebrate Types. Wiley, New York. 597p.
Ruppert EE, Fox RS, Barnes RB. 2004. Invertebrate Zoology, A functional evolutionary approach, 7 th ed. Brooks Cole Thomson, Belmont CA. 963 pp.
Supplies
Dissecting microscope
Compound microscope
Slides, coverslips
Living Ophioderma brevispina (or preserved)
Seawater
20-cm culture dish
Isotonic magnesium chloride
Paper clip
Carmine-seawater suspension
Bleach
Dissecting set with microdissecting tools
Plastic Pasteur pipet
Chalk dust
Preserved specimens are available from Carolina Biological (labeled “brittle stars”) and from Ward’s natural Science Establishment (labeled “Ophiura or similar genus”)