Invertebrate Anatomy OnLine
Notropis ©
Minnow
5jul2006
Copyright 2001 by
Richard Fox
Lander University
Preface
This is one of many exercises available from Invertebrate Anatomy OnLine , an Internet laboratory manual for courses in Invertebrate Zoology. Additional exercises, a glossary, and chapters on supplies and laboratory techniques are also available at this site. Terminology and phylogeny used in these exercises correspond to usage in the Invertebrate Zoology textbook by Ruppert, Fox, and Barnes (2004). Hyphenated figure callouts refer to figures in the textbook. Callouts that are not hyphenated refer to figures embedded in the exercise. The glossary includes terms from this textbook as well as the laboratory exercises.
Systematics
ChordataP, Metameria, Vertebrata sP, Osteichthyes C, Cypriniformes O, sO, Cyprinidae F (Fig 27-12, 29-32)
Chordata P
Chordata is characterized by a suite of apomorphies that include a dorsal hollow nerve cord, notochord, pharyngeal gill slits, and a post anal tail (Fig 29-1). The ancestor was a fishlike deuterostome that swam using alternating contractions of right and left longitudinal axial muscles to create undulations of the body. The flexible, incompressible notochord prevented these contractions from compressing the body while allowing lateral deflection. The chordate central nervous system is a hollow, median, longitudinal nerve cord formed in the embryo by an invagination of surface ectoderm whose original function was probably sensory reception. Paired pharyngeal gill slits connect the lumen of the pharynx with the exterior and originally functioned in suspension feeding with respiration being added later. A muscular tail posterior to the anus is, although commonplace in chordates, an unusual feature not found in other taxa. It is an extension of the axial musculature and is the chief locomotory organ. An additional apomorphy is the endostyle, a region of pharyngeal endoderm, that secretes iodated compounds, either mucus or hormones.
Vertebrata sP
Vertebrata, by far the largest and most diverse chordate taxon, includes three higher taxa of extant fishes (agnaths, cartilagenous fishes, and bony fishes) and four of tetrapods (amphibians, reptiles, birds, and mammals). Because it includes Vertebrata, Chordata, with about 40,000 species, is the third largest higher animal taxon, after Arthropoda and Mollusca. Vertebrate blood vessels have endothelia, seen elsewhere only in cephalopod molluscs. Mesenchymal neural crest cells are unique and are the precursors of many adult tissues. Stratified epithelia are present. Glomerular kidneys consisting of metanephridia and coelomic capsules are the excretory organs. Anteriorly dorsal hollow nerve cord is elaborated to form a three-part brain (forebrain, midbrain, hindbrain) to process sensory input from three primary sense capsules (olfactory, optic, otic). Erythrocytes with hemoglobin are universally present (with one exception). Vertebrates have an endoskeleton of cartilage or bone. Vertebrae, paired appendages, and jaws are present in most.
Osteichthyes C
Osteichthyes, the bony fishes, is by far the largest vertebrate higher taxon with about 20,000 extant species. Although bone is not an apomorphy of bony fishes, endochondral (bone preformed in cartilage) bone is. Other apomorphies include similarities in lateral line canals and similarities in the dermal bones of the operculum and pectoral girdle.
Laboratory Specimens
Small fishes of the family Cyprinidae are available alive and inexpensively at local tackle and bait shops and are ideal subjects for an introduction to both vertebrate and fish anatomy. A variety of species and genera are sold as fishing bait and any of these can be used for this exercise. Cyprinidae includes minnows, shiners, chubs, carp, and goldfish. Members of this family are the only fishes correctly referred to as minnows.
The exercise is written for use with fresh, recently sacrificed, specimens but preserved specimens can be used if necessary. Individuals about 6-10 cm in length should be used. Color descriptions refer to fresh specimens and will not apply to preserved material. Colors of some structures may vary from species to species. Bait minnows are sometimes held in water to which a blue fungicide has been added. This blue pigment may show up in unexpected places and alter the natural color. Externally it may fill the lateral line canals (where it improves visualization of the canals) or stain the gill filaments. It may also appear internally as purple fat, blue gonads, and a purple spleen.
The teaching staff will sacrifice specimens immediately prior to the laboratory period by placing them in carbonated water. The study should be conducted with a dissecting microscope in a small pan or culture dish.
External Anatomy
Place a fresh, but dead, minnow in a small dissecting pan of tapwater. Arrange the fish so it is lying on its side with the head pointed to the left, from your viewpoint, and its left side up, exposed to view. Wet the specimen periodically to prevent its drying but do not immerse it in water. It is especially important that the fins and scales remain moist. Throughout this study you should rinse the body with jets of water from a pipet or, better, a squirt bottle, to remove mucus or anything else that obscures your view.
Observe the overall appearance of the specimen without magnification. Minnows, like most fishes, have a streamlined, or fusiform, shape to facilitate efficient locomotion through an incompressible medium 800 times as dense as air (Fig 1). The body of most minnows is flattened slightly from side to side, or laterally compressed. The outer covering, or skin, including the scales is the integument.
Body Regions
The body is divided into three regions (Fig 1). The head extends from the anterior tip of the fish to the posterior edge of the gill cover. The trunk is the largest part of the body and extends from the posterior edge of the gill cover to the anus. The anus may not be readily visible but in most fishes is situated immediately anterior to the anal fin, which is the large unpaired fin on the posterior ventral edge of the body. The postanal tail is the third body region and the most posterior. It extends posteriorly from the anus to the end of the body but does not include the caudal fin.
Figure 1. A generalized minnow. Redrawn from Hubbs & Lagler (1958). Fish167L.gif
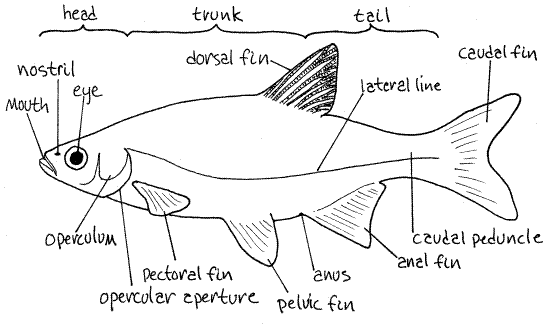
Head
The operculum, or gill cover, is a lateral plate of bone and soft tissue that covers the gill chamber (Fig 1). The opening immediately posterior to it is the common gill opening, oropercular aperture. The major bones of the gill cover are the opercle and preopercle. Both can be seen through the thin integument. The opercle is the larger and is the large flat bone making up the posterior portion of the gill cover. The preopercle is smaller, crescent shaped, and lies immediately posterior and ventral to the eye. Lift the posterior edge of the operculum to reveal the gill opening. Look into the gill chamber for a glimpse of the bright red gills. You will study them in more detail later.
The eye is located on the side of the head. Its large black pupil is surrounded by a silvery iris. The mouth is located at the anterior end of the head and in minnows is at the extreme anterior tip of the head, a position known as terminal (Fig 1). In many fishes, especially those that feed on the bottom, the mouth is a little posterior of the tip of the head and is on the ventral side of the head. Such a mouth is subterminal. A few fishes have the mouth on the upper surface of the head, posterior to the anterior tip, and these feed at the surface. The mouth of these fishes is said to be upturned.
Close the tips of your fine forceps and insert the combined points into the mouth then let the points separate to force the mouth open. Note the extent to which the mouth can expand when it opens. Most modern fishes feed using suction created by opening the mouth to create a negative pressure that sweeps water and food items into it.
Place the dissecting pan on the stage of the dissecting microscope and examine the mouth with low power (about 7-10x). Find the upper and lower jaws. The major bones in the upper jaw are the premaxillae and maxillae, one of each on each side. The premaxillae are anterior and median whereas the maxillae are on the sides, at the angle of the jaw. In some fishes these bones are clearly visible through the thin integument of the jaw but in minnows they cannot be seen.
There is probably an uninterrupted, horizontal, transverse groove between the upper lip and the remainder of the snout of your specimen. Fishes that have such a groove haveprotractile upper jaws. Some species have a bridge of tissue between the middle of the lip and the snout and the jaw cannot be protracted. Grasp the upper lip and pull it away from the snout, noticing that it is capable of moving far out in front of the snout when the mouth opens. This is what is meant by "protractile". Watch the premaxillae of the upper lip telescope into the snout as it moves back toward the snout.
The lower jaw is composed of many bones but the largest and most important is the dentary. In many fishes, but not minnows, the dentary bears teeth.
A nostril is located on each side between the eye and mouth and a little dorsal (Fig 1). Look at it with magnification. It consists of two openings separated by a flap of tissue (Fig 2). The flap will probably be lying over the posterior opening and must be lifted with the fine forceps or tiny needle to reveal the opening. The nostrils are lined with chemosensory epithelium and are blind pouches that do not connect with the buccal cavity. In fishes they have no respiratory function. Wash the mucus out of the nostril with a pipet or squirt bottle so you can see inside. Notice the papillae and ridges inside that increase the surface area for chemoreceptors. Insert the tip of a tiny needle into the posterior opening and slip it anteriorly until you can see it through the anterior opening, thus demonstrating the continuity between the two apertures. A ciliary current flows through the nostril.
Using magnification, observe the numerous chromatophores in the skin of the head (and the rest of the body as well). At about 20X you can easily discern the irregular star-shaped morphology of these pigments-containing cells. The fish can alter its color by enlarging or contracting the chromatophores. Some of them may be contracted into small black dots.
Look very closely at the surface of the head after rinsing it free of mucus with a strong jet of water from a squirt bottle. Careful examination will reveal rows of widely spaces pores. These are some of the many lateral line pores through which the lateral line sensory system communicates with the external world. The lateral line system is a series of water-filled canals with receptor cells for the detection of pressure waves in water (Fig 1).
Examine the ventral surface of the head with 7X. Grasp the ventral margin of the preopercle and move it laterally, away from the midline. Notice that it remains attached to the ventral middle of the head by a flexible, expansible branchiostegal membrane that extends from the edge of the gill cover to the throat region of the head. The branchiostegal membrane is the floor of the gill (branchial) chamber and is supported by slender curved bones known as branchiostegal rays.
Don't forget to keep your specimen wet. Empty the excess water into the sink occasionally.
Trunk
The trunk contains the coelomic, or abdominal, cavity and most of the viscera. Its walls consist mostly of axial muscles that participate in locomotion.
Hold the fish so its ventral edge is up and look closely at the region immediately anterior to the anal fin to find the anus (Fig 1). It may be very difficult to see. If so, remove all mucus from the area and use a bit of paper towel to dry the area. You can best find it by probing gently in an anterior direction with your fine forceps. The tip will slip into the anus and then you can see it. Just posterior to the anus are the openings of the urogenital system but it is unlikely that you will be able to demonstrate these.
Tail
The tail is the part of the body posterior to the anus and consists of the caudal peduncle and tail fin. Posterior to the anus the tail narrows markedly to form the caudal peduncle, to which the caudal fin (tail fin) is attached (Fig 1). The tail is composed almost entirely of axial muscles and skeleton which, along with the axial musculature of the trunk region, powers the caudal fin during swimming. The abdominal cavity and viscera do not extend posteriorly into the tail. The postanal tail is a chordate apomorphy.
Fins
Fishes have two types of fins, paired and unpaired (Fig 1). The two pairs of paired fins are homologous to the paired appendages of tetrapods. The paired fins are located laterally, on the sides of the body, whereas the unpaired fins are medial, on the midline. Fins consist of membranous sheets of tissue supported by either soft rays or hard spines or both. The spines and rays are controlled by muscles and can be raised, lowered, or moved to either side. Rays are flexible, jointed and often branched distally. Spines are usually stiff, pointed, unjointed, and unbranched.
Paired Fins
The pectoral fins are the anterior paired fins and are homologous to the forelimbs of tetrapods. They are always found immediately posterior to the opercular aperture and the skeleton of the pectoral girdle is attached to the posterior part of the skull. Use fine forceps to expand the fin so you can see its extended shape.
Look at a pectoral fin with magnification. Note that it is supported by numerous soft rays. Place a small strip of white paper behind the fin so you can see the rays better. Examine the rays at about 15X and note that they are jointed and distally branched, as is characteristic of rays.
The position of the pelvic fins varies widely with taxon and they can be almost anywhere just lateral to the ventral margin of the trunk. In minnows they will be close to the ventral midline and about halfway between the anus and the operculum. Notice that they are also supported by rays. The jointed nature of these rays may be easier to see than that of the pectoral fins.
Unpaired Fins
Most fishes have three or four unpaired fins. All are located on the midline, either dorsal, ventral, or posterior.
The dorsal fin is on the dorsal midline. In many fishes it is composed of two regions, an anterior spiny fin and a posterior soft rayed fin. The two may be coalesced, touching, or separate. In many other fishes only one dorsal fin is present and it is supported by soft rays. Cyprinids, such as the one you are dissecting, have only the single soft-rayed dorsal fin. Locate this fin on the dorsal midline of your specimen. It is located near the middle of the dorsum.
The caudal fin (tail fin) is the large fin at the posterior end of the body. It is often called the "tail" but not by ichthyologists. In almost all bony fishes it is symmetrical and is thus homocercal. Sharks and some primitive bony fishes have asymmetrical heterocercal caudal fins.
The anal fin is the only fin on the ventral midline and is located immediately posterior to the anus. In cyprinids it is supported by soft rays.
In some taxa (catfishes, salmon, and trout) an adipose fin is present on the dorsal midline posterior to the dorsal fin. It is a small fleshy lobe without rays, spines, or membrane.
Scales
Most bony fishes are covered over most of the body by dermal scales. These are usually small disks of flexible dermal bone covered by a thin layer of epidermis. Some parts of the body, such as the head, may be naked, without scales. Some taxa, such as catfishes, lack scales entirely.
Wet the surface of the trunk of your specimen and then look at it with about 10x magnification. Notice the overlapping pattern formed by the scales. Slip one tip of your fine forceps under the posterior edge of a scale and lift it. Grasp the scale and pull it free of the body.
Hold the single scale in the air above the fish, so the fish provides a contrasting background, and focus on it. Notice that its posterior, exposed, margin is circular whereas the covered anterior margin is more or less truncate.
Scales grow by deposition of new bone in concentric circles around the periphery of the scale. This results in a pattern of concentric circles known as circuli, which are easily seen in both the extracted scale and those still on the body. Examine the extracted scale at 30X to see the circuli.
Look at the surface of the scale you removed. There likely are chromatophores on its outer surface, offering you proof, in case you were skeptical, that the scales are internal with a layer of tissue outside of them.
Most bony fishes have one of two types of scales, either ctenoid or cycloid. In general, fishes with spiny dorsal fins have ctenoid scales whereas those with soft dorsal fins have cycloid scales. Which type would you expect to find in minnows?
Ctenoid scales have a patch of tiny teeth on the anterior, covered portion of the scale. These teeth are lacking in cycloid scales. Examine your extracted scale at 30x to test your prediction.
Scales are absent from all or part of the head. Look at the head of your specimen. Is it naked or scaled?
A row of special lateral line scales extends the length of the body on each side of the fish from operculum to caudal fin. These scales form the lateral line (Fig 1) and lie above the lateral line canal. Each of the scales in this line is perforated by a canal and pore. A major sensory system, the lateral line system, lies beneath the line and communicates with the external environment through these canals and pores. Earlier in the study you saw another portion of the lateral line system on the head.
Look at the side of your specimen without magnification and find the lateral line. It starts at the posterior edge of the operculum and runs posteriorly across the trunk and caudal peduncle to end at the caudal fin.
Look at the lateral line with 20X magnification. If you have allowed your scales to dry out at any time during the exercise the canals in the scales may contain air and this increases their visibility. Even without the air, however, they are easy to see once you find the lateral line. With fine forceps remove one of the scales from the lateral line and observe it as you did the ordinary scale earlier. Find the canal running lengthwise through the scale and the pore at the distal end of the canal. The pore is in the medial side of the scale, not the lateral as you may have assumed. You can probably slip the tip of a tiny needle into it to demonstrate its presence. Remember to try this on the medial, not lateral side.
Internal Anatomy
Pharynx
The pharynx is a region of the gut but because it is close to the outside of the animal and because it will be destroyed by later procedures, it is preferable to study it now, prior to opening the body.
The pharynx is the gut tube between the buccal cavity and the esophagus and is defined as the region of the gut with pharyngeal gill slits and gill arches. Pharyngeal gill slits are one of the chordate apomorphies. The pharynx and gill arches lie inside the operculum which covers and protects them. Gill arches are U-shaped columns of tissue that form the walls of the pharynx. Each arch contains a skeleton, cranial nerve, muscles, and an aortic arch in addition to gill filaments. The gill slits are the openings between successive arches. Water enters the mouth, flows posteriorly to the pharynx, passes laterally through the gill slits and over the gill filaments into the gill chamber, and then exits through the opercular aperture behind the operculum. The vascularized gill filaments are the site of gas exchange. Although the original function of gill slits was suspension feeding their role in most vertebrates is respiratory.
At about 7X magnification, lift the operculum and examine the gill chamber (= branchial chamber) within (branch is Latin for gill) (Fig 2). This will require that you force the gill cover anteriorly and bend it just posterior to the eye. It will bend easily near its middle but you must lift the entire operculum. Hold the gill cover out of the way with the fingers of one hand while you explore with the fine forceps in your other hand. The gill chamber is exposed now. In it are four gill arches (= visceral arches) but you can only see one of them at present (Fig 2). The one you see hides the other three, which are posterior and medial to it. Bony fishes have a total of seven visceral arches (I-VII) but the first two are incorporated in the jaws and no longer bear gill filaments or function in respiration. The seventh arch forms the posterior wall of the gill chamber and like I and II, does not function in respiration. Thus the gill chamber contains only four of the original seven arches. These four are the only arches that continue to be respiratory in function and for convenience they are numbered 1-4 (even though they are really III-VI). Wash the mucus off the gill arch and out of the gill chamber.
Figure 2. The head of a cyprinid, the creek chub, Semotilus, with the operculum removed to expose the gill chamber. The four respiratory gill arches are numbered 1-4. The non-respiratory tooth-bearing arch 5 is also numbered although the teeth are not visible. Redrawn from Hubbs and Lagler (1958). Fish168L.gif
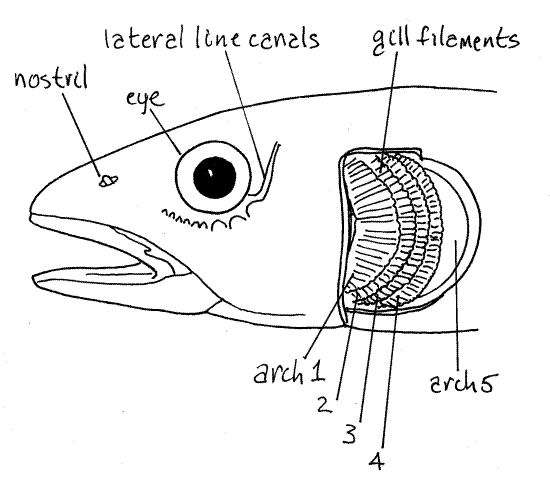
Look at the first respiratory gill arch (#1) (Fig 2). Only half of it is visible to you on this side. The other half is on the other side of the fish. The part of the arch you see is roughly "C" shaped with the concavity of the "C" facing anteriorly. The outside curve of the "C" bears the red (or pink) gills. These are the gas exchange surfaces of the fish. Look at the gill with 20X magnification to see that it is composed of a set of parallel gill filaments that extend outward from the arch. If you look very closely with careful focusing you will see that the filaments bear tinygill lamellae extending from their surfaces. Together the filaments and lamellae provide the fish with the necessary surface area for gas exchange. There are two rows of filaments on each arch.
The inside curve of the gill arch bears a set of evenly spaced, long, pointed gill rakers. These play various roles in feeding in different species. In some taxa they are so numerous and so closely spaced they can be used as a filter to remove fine particulate food from the water. In minnows they are used to prevent small crustaceans, insects, and other prey from escaping the gill chamber by swimming through the gill slits. They also protect the delicate gill filaments from damage by these prey animals. A second row of rakers is present on the medial surface of each arch.
Lift the first gill arch to expose the second, then the third, and finally the fourth (Fig 2). The fifth gill arch forms the posterior wall of the gill chamber and does not bear filaments but it may have rakers or large pharyngeal teeth. After you have completed your study of the pericardial cavity and heart, near the end of the exercise, you can return to the gill chamber and remove the fifth arch and examine these teeth.
The gaps between successive gill arches are the gill slits. They are passageways from the lumen of the pharynx to the gill chamber outside the arches and water flows through them. Five gill slits are on each side.
In some fishes a vestigial gill, really just a patch of filaments, occurs on the inner surface of the operculum. This is a pseudobranch (false gill) and there is probably one in your specimen. Look for it.
Buccal Cavity
" Use your heavy scissors (DO NOT use your delicate iridectomy scissors for this) to cut posteriorly from each corner of the mouth between the branchiostegal rays and the margin of the operculum. This will allow you to pull the floor of the mouth away from the head and examine the buccal cavity and pharynx.
The mouth opens into the buccal cavity (= mouth cavity) which is the first chamber of the digestive tract. In fishes teeth are found on a variety ob bones depending on taxon. The upper and lower jaws are customary locations but almost any bone in the buccal or pharyngeal cavities can be tooth bearing. Search the interior of the buccal cavity at 7x, then 20x, for teeth. Minnows do not have teeth in the buccal cavity but, as you know, they do in the pharynx.
The wide, fleshy, median, longitudinal ridge on the floor of the buccal cavity is the tongue. It is attached to the floor of the mouth along its entire length and is not protrusible.
Without magnification, look into the open mouth and find the gill arches and gill slits in the pharynx on the right and left sides. You have seen them earlier from a different vantage point. Use your fine forceps to separate them so they can be counted. The level of the first gill slit is approximately the line of separation between the buccal cavity and the pharynx.
Peritoneal Cavity and Viscera
The coelomic cavity of fishes is divided into a large peritoneal, or abdominal, cavity and a much smaller pericardial cavity. The peritoneal cavity contains the viscera whereas the pericardial cavity contains the heart. There is no pleural cavity, even in fishes with lungs. The peritoneal cavity occupies most of the ventral half of the trunk whereas the pericardial cavity is anterior and near the ventral margin just posterior to the operculum and anterior to the peritoneal cavity.
" Hold the fish in one hand with the venter up, facing you. Insert the tip of one blade of your fine (iridectomy) scissors into the anus and cut anteriorly, exactly on the ventral midline. The cut should be no deeper than is necessary to cut through the body wall and open the peritoneal cavity. After you have cut about 5 mm, you can spread the sides of the cut apart to see if you have penetrated the cavity or if you need to cut deeper. Extend this incision anteriorly until you reach the level of the pectoral fins. You are approaching the pericardial cavity.
Continue the incision, very carefully now, and still shallow and on the midline, anteriorly to the gill chamber. You are now opening the pericardial cavity. Use your heavy scissors to cut through the bone in the posterior wall of the gill chamber. Never use the iridectomy scissors to cut bone. The peritoneal cavity is now open but its contents are not yet accessible to you.
At the anterior end of the peritoneal cavity the delicate transverse septum extends from side to side and forms the anterior wall of the cavity. It will have been damaged, but hopefully not destroyed, by the midventral incision. The pericardial cavity is anterior to the transverse septum and the peritoneal cavity is posterior to it. The pericardial cavity and heart are enclosed in a capsule of bright white membrane (peritoneum) between the transverse septum and the gill chamber. The pericardium should be left intact for the time being while you concentrate on the peritoneal cavity.
Peritonea
Place the fish in the dissecting pan on the stage of the microscope at the lowest power. Hold the two sides of the incision open with one hand and look into the peritoneal cavity. This is awkward but it's the best way to dissect a laterally compressed fish. Look around inside the cavity and find the peritoneum. Peritoneum is the epithelium that lines the coelomic cavity. It is a thin sheet of squamous epithelium that coversall surfaces in the cavity. Where it covers organs it is known as visceral peritoneum whereas that covering the wall of the cavity is parietal, orsomatic peritoneum. Its color varies with taxon and may be bright white, silver, or black. Some fishes have a profusion of black chromatophores in the peritoneum.
Fat Deposits
The white frothy tissue that covers most surfaces is adipose connective tissue with fat deposits. Look closely and you will see that it is composed of innumerable spherical fat dropletsthat are responsible for the foamy appearance.
" You must remove the body wall on the left side to expose the viscera to view. Look into the peritoneal cavity and push the organs away from the left body wall. You may need to tear some peritoneum. Insert the fine point of your coarse (not iridectomy) scissors into the posterior end of the cavity, just anterior to the anus. Cut dorsally, and slightly posteriorly, through the body wall. This cut will be perpendicular to the initial midventral cut. Watch, with magnification, what your scissors are doing and be sure you cut only the body wall. Its white muscle is easily recognized. Don’t cut anything red.
Continue cutting dorsally until you see red tissue lying beside the dorsal midline of the peritoneal cavity. Do not cut into the red tissue (it is the kidney). Upon reaching the kidney, change the direction of the incision and extend it anteriorly, parallel to the margin of the kidney. Push organs to the right side of the fish to avoid cutting them. You may remove fat or peritoneum if it gets in the way but leave all other organs intact. When the incision reaches the transverse septum, change directions again and cut ventrally to connect with your original midventral incision. The left body wall has now been removed and can be discarded. Rinse the debris from the abdominal cavity and keep the cavity moist.
Kidney
Relocate the red tissue lying beside the dorsal midline in the roof of the peritoneal cavity. This is the kidney. Fish kidneys are narrow, straplike, and extend the entire length of the abdominal cavity. In early vertebrates the kidneys extended the length of the peritoneal cavity with a pair of nephrons in each segment. The bean-shaped kidneys of mammals are derived from that ancestral condition. The kidney is greatly expanded at the anterior end and here is called the head kidney. The expansion is due to large blood sinuses in the tissue.
Swim Bladder
Ventral to the kidney is the conspicuous swim bladder. It, and the kidney, are retroperitoneal meaning they are outside (retro = behind) the peritoneal cavity. The swim bladder looks like a balloon and is. It is divided into anterior and posterior chambers by a narrow isthmus. Gas is secreted from the blood by the epithelium of the anterior chamber. In the posterior chamber gas is reabsorbed. By balancing secretion and absorption, the fish can control the volume of the bladder and maintain neutral buoyancy, thus its position, at any depth in the water column.
Rub some of the epithelium off the swim bladder. The bladder will look even more like a balloon now. You can free the posterior end of the bladder and lift it partly out of the peritoneal cavity to get a better look at it. Note its consistency and texture; ie balloon-like and air-filled. A pneumatic duct can be seen running to the posterior chamber of the swim bladder. Tuck the bladder back into the cavity.
Gonads
Look ventral and lateral to the swim bladder to find the gonad. The size of this organ varies widely depending on reproductive condition. Mature ovaries are larger than mature testes. At the height of the breeding season the ovary of some fishes can account for up to 70% of the body weight. If your specimen is a female the gonad is, of course, an ovary, and will probably contain recognizable spherical eggs. It may be pale gray or bluish gray. A testis will contain sperm and will be white with no recognizable gametes in it. Sperm are too small to be seen at this magnification.
>1a. Make a wetmount with a small piece of macerated gonad and examine it with the compound microscope. Eggs will be large nucleated spheres. Sperm will be tiny flagellated cells.<
Digestive System
Most of the remaining viscera are either the gut or associated organs. The gut is often heavily invested with fat deposits making it impossible to see its shape without removing the fat. This should be done but it is tedious and will require some time. Be careful that you do not cut the gut while removing fat.
The large liver is pale brownish pink and its base is attached to the transverse septum. Three long narrow lobes extend posteriorly from the base and are attached to the mass of fat around the gut. Push the viscera to the left and look on the right side. Here you will see two of the liver lobes. One is approximately on the ventral midline and the other is to the right of it. A large yellow-green discoloration marks the position of the gall bladder near the base of the right lobe. Return the viscera to their original position and find the left lobe.
The elongate, coral-pink or dark brown spleen lies between the gut and the left liver lobe.
Clean the fat deposits from the gut as best you can. This is best accomplished by lifting small lobes of fat with your fine forceps and then cutting them free close to the gut tube with the iridectomy scissors. The gut itself is very pale brownish pink (much paler than the liver) and for most of its length is tubular. In the vicinity of the liver the gut is coiled into an S-shaped loop but posteriorly it runs straight to the anus.
The esophagus extends posteriorly from the pharynx and passes through the transverse septum where it is hidden by the liver. It joins the stomach after passing through the liver. The stomach is surrounded by fat deposits which must be removed. The duodenum exits the stomach and then reverses direction and heads anteriorly again as the intestine. Upon reaching the anterior end of the peritoneal cavity it again reverses direction and extends posteriorly to the anus.
Pericardial Cavity and Heart
" Use your iridectomy scissors to open the pericardial cavity by making a midventral incision through the bright white pericardial peritoneum surrounding it. Like the peritoneal cavity, it may be filled with fat deposits which must be carefully removed. Be very careful in the extreme posterior region of the pericardial cavity adjacent to the transverse septum. The sinus venosus is located here but is hard to see and easily damaged. Three of the four chambers of the heart are large, thick-walled and readily visible but the sinus venosus is thin-walled and transparent and can be destroyed before you see it. Rinse the pericardial cavity with a squirt (gentle) of water.
Although fishes have a four-chambered heart they are not the same four chambers found in a mammalian heart. The fish circulatory pattern is a single circuit and the four chambers are in series, one after the other, rather than parallel. The sinus venosus is the first chamber of the heart. It is triangular and transparent and lies against the anterior side of the transverse septum in the posterior pericardial cavity. It receives all returning venous blood.
The atrium is next in line and much easier to see. It is pink and lies mostly on the left side of the pericardial cavity. It receives, and accumulates briefly, blood from the sinus venosus before passing it on to the ventricle.
The ventricle is a large, thick-walled, muscular, beige chamber occupying a prominent position in the middle of the pericardium. Contractions of the ventricle pressurize the blood and force it anteriorly.
The ventricle empties into the conus arteriosus. This large, bulbous, pink chamber lies anterior to the ventricle. The slender ventral aorta can be seen exiting its anterior extremity and disappearing into the anterior wall of the pericardial cavity on its way to the gill chamber.
In the gill chamber, the ventral aorta branches into five pairs of aortic arches which take blood to the gill arches where it is oxygenated. Oxygenated blood from the gills is distributed to the body by branches of the dorsal aorta, which originates above the gills. Blood then returns to the sinus venosus by means of a venous system consisting of renal portal, hepatic portal, and cardinal systems.
Pharyngeal Teeth
Return to the gill chamber and relocate the fifth gill arch. It is a heavy bone forming the posterior wall of the branchial chamber and is immediately anterior to the pericardial cavity. Use your iridectomy scissors to cut the soft tissue holding it in place. DO NOT try to cut the arch itself with these scissors. When the arch is free of most of its soft connections, grasp it with your medium forceps and twist it out of the body. Do not use your fine forceps for this.
Place the arch in a small culture dish of water. With forceps remove all soft tissue so you can see the bony arch and its pharyngeal teeth. These large, robust teeth are characteristic of minnows and are used to macerate food before it moves farther posteriorly to the stomach. Minnows have no teeth on the jaws or in the buccal cavity.
References
Chiasson RB, Radke WJ. 1991. Laboratory anatomy of the perch, 4 th ed. Wm C. Brown, Dubuque.
Hubbs CL, Lagler KF. 1958. Fishes of the Great Lakes region. Cranbrook Institute of Science Bull 26, Bloomfield Hills, MI. 213 pp.
Page LM, Burr BM. 1991. Freshwater fishes. Houghton Mifflin, Boston.
Ruppert EE, Fox RS, Barnes RB. 2004. Invertebrate Zoology, A functional evolutionary approach, 7 th ed. Brooks Cole Thomson, Belmont CA. 963 pp.
Supplies
Dissecting microscope
Compound microscope
Slides and coverslips
Dissecting set with microdissecting tools
Fresh or preserved minnow
Small sardine can dissecting pan
Club soda
Squirt bottle of tapwater
6-cm culture dish