Invertebrate Anatomy OnLine
Nereis virens ©
Ragworm
28may2007
Copyright 2001 by
Richard Fox
Lander University
Preface
This is one of many exercises available from Invertebrate Anatomy OnLine , an Internet laboratory manual for courses in Invertebrate Zoology. Additional exercises, a glossary, and chapters on supplies and laboratory techniques are also available at this site. Terminology and phylogeny used in these exercises correspond to usage in the Invertebrate Zoology textbook by Ruppert, Fox, and Barnes (2004). Hyphenated figure callouts refer to figures in the textbook. Callouts that are not hyphenated refer to figures embedded in the exercise. The glossary includes terms from this textbook as well as the laboratory exercises.
Systematics
Annelida P, Polychaeta C, Palpata, Aciculata, Phyllodocida O, Nereidacea SF, Nereidae F (Fig 13-7A)
Annelida P
Annelida consists of the segmented worms in the major taxa Polychaeta (bristleworms), Oligochaeta (earthworms and relatives), Branchiobdellida (crayfish ectosymbionts), and Hirudinea (leeches) with a total of about 12,000 known species in marine, freshwater, and terrestrial environments. The segmented body is composed of an anterior prostomium, a linear series of similar segments, and a posterior pygidium. The prostomium and pygidium are derived from anterior and posterior ends of the larva whereas the intervening segments arise through mitotic activity of mesodermal cells in the pygidium.
The body wall consists of a collagenous cuticle secreted by the monolayered epidermis. A connective tissue dermis lies beneath the epidermis. The coelom is lined by a peritoneum which may be specialized to form the body wall muscles. Most annelids have chitinous bristles, or chaetae, secreted by epidermal cells, that project from the body. The coelom is large, segmentally compartmented, lined by peritoneum, and well developed in polychaetes and oligochaetes but reduced in leeches. Successive coelomic spaces are separated by transverse bulkheads known as septa which consist of double layers of peritoneum with connective tissue in between. The right and left sides of each segmental coelom are separated by longitudinal mesenteries which, like septa, are double layers of peritoneum with connective tissue between.
The gut is a straight, regionally specialized tube that begins at the mouth at the anterior end and extends for the length of the body to end at the anus on the pygidium. It penetrates each septum and is supported by dorsal and ventral mesenteries. Like that of most invertebrates, the gut consists of ectodermal foregut, endodermal midgut, and ectodermal hindgut. The nervous system consists of a dorsal brain in or near the prostomium, a pair of circumpharyngeal connectives around the anterior gut, and a double, ventral nerve cord with paired segmental ganglia and nerves. The hemal system of most annelids is a set of tubular vessels, some of which are contractile and serve as hearts. The hemal system is absent or greatly reduced in leeches. The system includes a dorsal longitudinal vessel above the gut in which blood moves anteriorly, a ventral longitudinal vessel below the gut, in which blood moves posteriorly, and paired segmental vessels that connect the dorsal and ventral vessels. The digestive, hemal, and nervous systems are continuous and pass through the segments.
Respiration is accomplished in a variety of ways. In some, the general body surface is sufficient but gills are present in most polychaetes, many leeches, and a few oligochaetes. Excretory organs are metanephridia or protonephridia and typically one pair is present in each segment. These osmoregulatory organs are best developed in freshwater and terrestrial species. The sexes are separate in polychaetes but oligochaetes and leeches are hermaphroditic. In the ancestral condition paired submesothelial clusters of germ cells were present in each segment and released developing gametes into the coelom. In derived taxa reproductive functions tend to be confined to a few specialized genital segments. Gametes mature in the coelom or its derivatives and fertilization is external. Gametes are shed through ducts derived from metanephridia or by rupture of the body wall. Spiral cleavage follows fertilization. Clonal reproduction is common.
Polychaeta C
Polychaeta is a large (8000 species) and diverse taxon of marine annelids thought to be the most primitive of the annelid taxa and the most like the ancestral annelid. The body of a typical polychaete is divided into segments, each of which bears a pair of fleshy appendages, or parapodia. The head is often equipped with abundant, well-developed sense organs. The anterior gut is muscular, sometimes eversible, and frequently equipped with chitinous jaws. Polychaetes are gonochoric and gametes ripen in the coelom from which they are shed through ducts or by rupture of the body wall.
Palpata
The prostomium has a pair of sensory palps. These are lacking in the sister taxon, Scolicida.
Aciculata
Aciculata is a large taxon containing many worms well-known to marine biologists and invertebrate zoology students. The parapodia are well-developed and biramous. The prostomium has antennae. Aciculates were once known as “errant” polychaetes because they are active and mobile.
Phyllodocida O
These are the polychaetes with a muscular eversible pharynx.
Laboratory Specimens
Nereids are typically used in textbooks (e.g. Fig 13-10, 13-12A, 13-14, 13-18B, 13-26D, 13-30A, 13-33A,B), laboratories, and lectures as examples of basic annelid anatomy because they are relatively little modified from, and are thought to exemplify, the ancestral polychaete more closely that other Recent taxa. Nereidae is homogeneous and for our purposes there is little variation between its species. The exercise is written specifically for Nereis virens but can be used equally well with any other large nereid. Living or preserved specimens can be used but, as always, living specimens are preferable.
Nereis virens , the ragworm, clamworm, or sandworm, is a large species that may reach almost a meter in length but is usually considerably smaller, around 20-40 cm. It is a shallow-water, marine, benthic species occurring from the shoreline to depths of about 150 m. It occurs from Newfoundland to Virginia in North America and in northern Europe from Norway and Iceland south to France. It is common on the northeastern Atlantic coast of North America and, along with the bloodworm, Glycera, supports a commercial bait fishery in coastal Maine and the Canadian Maritime Provinces. Inexpensive living specimens are available from coastal bait stores or by mail from bait wholesalers in the northeast. Biological supply companies also provide these worms, either living or preserved. Prepared slides of body cross sections and wholemounts of parapodia are also available from supply companies.
Large Nereis require about three hours to be fully relaxed by isotonic magnesium chloride. They are difficult to handle before they are completely relaxed so the specimens should be placed in the relaxant early by the teaching staff. If possible, a separate unrelaxed specimen should be used for study of behavior and movement.
Anatomy
External Anatomy
Examine a living or preserved Nereis in a dissecting pan covered with isotonic magnesium chloride (living) or tapwater (preserved). The dissection should be conducted under magnification on the stage of the dissecting microscope.
Body
Observe the elongate, vermiform shape and the bilateral symmetry of the worm. The long axis of the body is the antero-posterior axis and is the axis of symmetry. The median sagittal plane, including the axis of symmetry, is the plane of symmetry that divides the worm into mirror image right and left sides (Fig 9-1A). The worm consists of a small anterior prostomium, a tiny posterior pygidium, and a very long segmented trunk between the prostomium and pygidium (Fig 13-1, 13-6). (The posterior end of the worm, with the pygidium, is often damaged or lost during collection. It may be missing in your specimen.) The body is slightly depressed dorsoventrally and is divided into numerous (as many as 200) successive segments. Externally each segment is separated from its neighbors by a shallow circumferential groove. Each segment bears a pair of fleshy, lateral appendages, the parapodia (Fig 2, 13-6A).
The body is covered by an iridescent, collagenous cuticle secreted by the epidermis beneath it. The iridescence is caused by microscopic striations which diffract light. The dorsal blood vessel may be visible through the body wall as a dark, median, longitudinal line, especially in living specimens.
If you have a living specimen, observe the blood vessel under magnification. Watch as prograde waves of peristaltic contractions move blood from posterior to anterior in this vessel. Watch as the vessel empties and then gradually refills. Smaller blood vessels in the body wall and appendages are also visible through the integument of living animals.
Prostomium
The head, with the mouth and an abundance of sense organs, is at the extreme anterior end. Study the head with low power (5-10X). It is composed of a dorsal, anterior prostomiumwith which several anterior segments have fused (Fig 1, 13-10). The prostomium itself is a smallish, flattened, pyriform (in dorsal view) plate on the dorsal surface of the anterior end of the worm. It is situated over (dorsal to) the very large, puckered, ventral mouth but does not extend around the sides of the mouth.
Figure 1. Dorsal view of the head of Nereis virens. Poly76La.gif
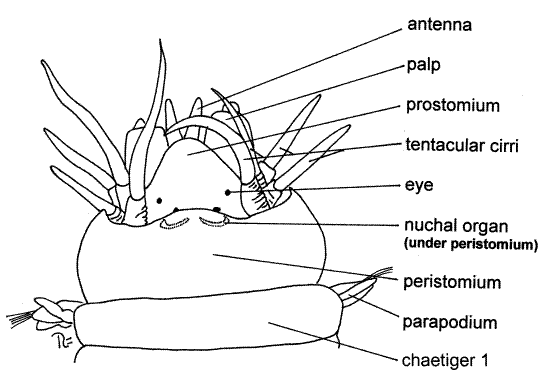
The prostomium bears a pair of small, short antennae attached to its anterior margin, on either side of the midline (Fig 1, 13-10A). A pair of much larger, fleshy palps extends anterolaterally and slightly ventrally from the sides of the prostomium. Each palp is composed of two sections, or articles. The proximal article is very thick and fat whereas the distal one is tiny. Four black or reddish eyes are located on the posterior dorsal surface of the prostomium at the four corners of an imaginary trapezoid. A pair of crescent-shaped, chemosensory nuchal organs is located near the posterior border of the prostomium. They may be partially or wholly obscured by the overhang of the adjacent segment (Fig 1).
If the head of your specimen seems to differ markedly from this description or from Figure 1, it is probably because the pharynx is partially or wholly protruded from the mouth (Fig 13-10C).
Under normal circumstances with the pharynx retracted, the mouth is located ventral to the prostomium as described earlier and no part of the body is anterior to the prostomium (Fig 13-10B). When feeding, the anterior end of the gut (the pharynx) is turned inside out and everted from the mouth (Fig 13-10C). With the anterior gut everted, the pharynx extends well anterior to the overlying prostomium, which is reflected dorsally and posteriorly, and the appearance of the anterior end of the worm is changed dramatically.
The inner walls of the pharynx bear teeth and jaws. Eversion of the pharynx during feeding brings the jaws and teeth to the outside where they can be used to capture food. Eversion may also be caused by stress such as that resulting from immersion in magnesium chloride or formalin.
If the pharynx of your specimen is not everted, or is only partly everted, reach into the mouth with your fine forceps and grasp the tough inner walls of the pharynx and gently pull the pharynx from the mouth. This will not work unless the animal is fully relaxed. It may or may not work on preserved specimens. If you cannot evert the pahrynx of your specimen, look instead at a demonstration specimen with a fully everted pharynx.
When completely extended, the two black, serrated, chitinous jaws are exposed at what is now the anterior end of the worm (Fig 13-10C). They form a pincers that opens during eversion but closes during retraction to pull whatever is caught into the mouth.
Notice that the pharynx is lined with an iridescent cuticle , as is the body surface. The pharynx, like the animal’s surface, is covered by cuticle-secreting epidermis. The pharynx is part of the foregut, which is derived from the ectodermal stomodeum of the embryo.
The exposed wall of the everted pharynx bears small chitinous teeth, or denticles, in the genus Nereis (Fig 13-10C). (In other nereid genera there may be soft papillae, comb-like teeth, or nothing on the pharyngeal wall.) When you have finished examining the everted pharynx, use the blunt end of the handle of your microneedle to push it back into the mouth to its resting position. (If you are looking at a demonstration specimen, leave the pharynx as it is.)
The peristomium is a complete ring immediately posterior to the prostomium (Fig 1, 13-10A, B). It completely encircles the mouth. Although the peristomium is probably a true segment, it is not typical and it lacks typical parapodia. Instead of parapodia, it bears four pairs of long, whip-like, sensory tentacular cirri. The peristomium of Nereis is thought to be derived from two fused anterior segments and the four pairs of tentacular cirri are derived from their parapodia. The peristomium is said to be apodous because it lacks typical feet, or parapodia. Some zoologists believe the peristomium is not a segment.
Trunk
The remaining segments resemble each other and have paired lateral parapodia which bear fleshy lobes with bristle-like setae. These segments are called chaetigers because they bear chaetae. The peristomium has no chaetae and thus is not a chaetiger but the other segments are.
Parapodia
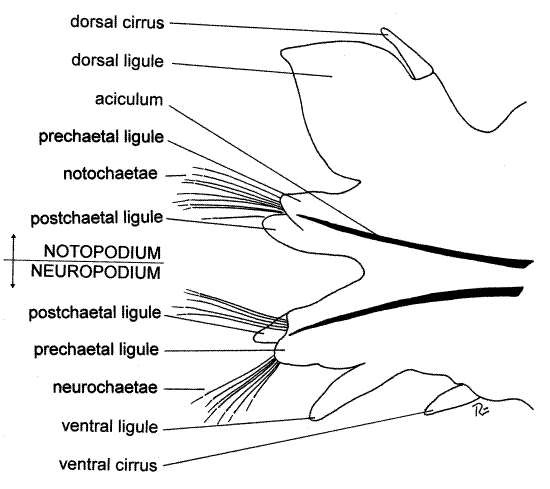
Look at an anterior parapodium from the region of about segment 20 (Fig 2). The parapodia function in locomotion but are also important gas exchange surfaces. They have well-developed musculature and are heavily vascularized. Under 10-15X orient the parapodium with fine forceps and a microneedle so you can see its anterior face. A typical polychaete parapodium is composed of two major branches, or rami, and accordingly is said to be biramous (Fig 2). The dorsal ramus is the notopodium (noto = back, pod = foot) and the ventral ramus is the neuropodium (neuro alludes to the nerve cord, which is ventral). Each ramus bears clusters of chitinous bristles, or chaetae.
" >1a. For a closer look at parapodial anatomy, use your fine scissors to remove the parapodium you just examined, make a wetmount of the entire parapodium, and study it with low power of the compound microscope (Fig 2). Increase the light intensity as necessary to view this opaque object.
Verify that the parapodium is composed of two rami. Find the dorsal notopodium and the ventral neuropodium. Each ramus is composed of smaller lobes, or ligules. The notopodium has a small, pointed, sensory dorsal cirrus on its dorsal margin. The notopodium is divided into three ligules (Fig 2). The dorsal ligule is by far the largest and most conspicuous of the three and is leaf-shaped. It is a gill used for gas exchange and is heavily vascularized (Fig 13-25A). The dorsal cirrus is located on the dorsal border of the dorsal ligule. Ventral to the dorsal ligule are two much smaller ligules associated with a bundle of chitinous chaetae. These are the notochaetae. The prechaetal ligule is anterior to the bundle and the postchaetal ligule is posterior to it. Look at the chaetae with higher power and notice that they are jointed, or compound, chaetae, each being composed of two articulated pieces (Fig 13-4A,C).
The ventral ramus of the parapodium is the neuropodium. It is smaller than the notopodium and bears neurochaetae, three small ligules, and a small ventral cirrus on its ventral margin. Are the neurosetae compound?
Each parapodium is supported by two internal, black chitinous rods called acicula, one in the notopodium and another in the neuropodium. If you are using a wetmount you should have no difficulty seeing the acicula if the parapodium is thin enough to transmit light. If it is too thick, press on the coverslip above the parapodium with the handle of your microneedle to squash the tissue a little. Do not press too hard or you may break the coverslip. If you are observing an entire specimen, pinch the sides of the parapodium together with forceps and you will be able to see these large, black, modified setae through the integument of the parapodium. <
Figure 2. Anterior view of the right parapodium of chaetiger 20 of Neereis virens. poly77La.gif
Pygidium
The posterior end of the worm is the pygidium, which is fused with the posteriormost true segment to form a tiny tail. The pygidium, like the prostomium, is not a segment. The segment fused with it has lost all parts of its two parapodia except for their two ventral cirri which form two long anal cirri. The anus is a large opening dorsal to the anal cirri. The pygidium, cirri, and some posterior segments are often lost when the animals are collected or handled.
Internal Anatomy
" Position the worm ventral side down in a long, narrow dissecting pan (an aluminum ice tray with a wax bottom makes a good worm pan). Place the anterior end of the worm in the approximate center of the pan. Most of your attention will be focused on this part of the worm. Before you pin the worm to the wax, be sure its head is at one end of the pan where it can be viewed with the dissecting microscope.
When you are satisfied with the position of the worm, temporarily anchor it to the wax by inserting a #1 insect pin at a 45 ° angle through the base of each of the parapodia of chaetiger 25 (approximately). Extend the anterior end of the worm toward one end of the pan from this anchor. Make sure it does not extend beyond the end of the pan. Relocate the worm and pins if it does.
Working under magnification, pinch the dorsolateral body wall of segment 25 (or thereabouts) with your fine forceps (a little to the side of the midline) to make a fold of the body wall. Use fine scissors to cut through the fold but be careful to avoid cutting across the midline and dorsal blood vessel. Make the cut entirely through the body wall. Insert the tip of the scissors into the opening and cut anteriorly through the body wall close to, but not on, the midline. This will open the coelom from which coelomic fluid will escape.
>1b. If you have a living specimen make a slide of the coelomic fluid. As you begin making the cut anteriorly, use a pipet to collect a little of the escaping cloudy coelomic fluid and make a wetmount with it. Avoid getting blood or gut contents in the sample. Set it aside for now (but don’t let it dry out) and return to it when you have finished opening and pinning the worm. The coelomic fluid contains abundant amoebocytes with filopodia. Your worm may also have gametes in the coelomic fluid. Developing gametes are released from the peritoneum into the coelom where they complete maturation. Look for eggs or spermatozoa, but not both, in these gonochoric animals. <
Extend the longitudinal incision from segment 25 to the posterior edge of the prostomium. This incision should lie beside the dorsal blood vessel but should not cross the midline or cut that vessel. This is especially important if you are dissecting a living animal. In Nereis this vessel adheres to the body wall (rather than to the gut wall as it does in Lumbricus) and it is best to leave it attached to the body wall. This means, of course, that you will have to cut its connections with the gut and viscera as you open the animal. Note the connections of the blood vessels before you cut them.
Figure 3. Dorsal view of the dissected anterior end of Nereis virens. Poly78La.gif
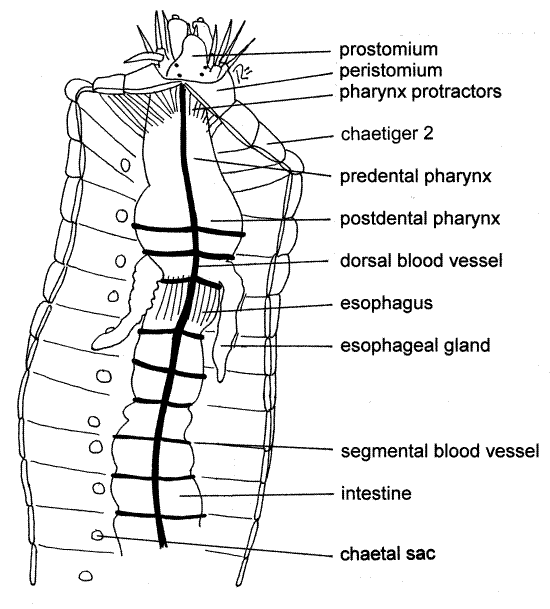
As you cut anteriorly, you will notice that diaphanous transverse partitions, the septa, separate the coelomic space of each segment from that of adjacent segment (Fig 3). Ventral to the gut the septa are incomplete and they do not form complete bulkheads between segments. Under magnification use fine scissors to cut the septa as you make the longitudinal incision. After cutting them, reflect the lateral body walls and pin them (the walls) flat to the wax of the dissecting pan. Use #1 insect pins inserted at 45 ° angles. The dorsal blood vessel, remember, should remain with the body wall.
When you reach the vicinity of segment 10, you will notice large muscles running from the gut tube to the dorsal body wall. These are the pharynx retractor muscles (but they do not originate on the pharynx). Try to leave them intact for the present.
Coelom and Body Wall
With the animal open, pinned, and completely immersed in fluid, look first at the body cavity which you have just revealed (Fig 3, 13-13A). It is the coelom. It is lined by a very thin mesodermal epithelium, the peritoneum. The inner surface of the body wall is covered by somatic peritoneum whereas the organs are invested with splanchnic peritoneum. In some areas specialized splanchnic peritoneum known as chlorogogen tissue has a yellowish color (in life).
Chlorogogen tissue, usually yellowish and a characteristic feature of annelid peritoneum, functions metabolically like the vertebrate liver. It stores glucose as glycogen and releases it when needed, synthesizes hemoglobin, detoxifies toxins, deamifies amino acids to produces ammonia and synthesize urea, and stores lipids.
Most of the thickness of the body wall is the conspicuous longitudinal muscles, which occur in four bundles (Fig 4). Of them, the two dorsolateral bundles are easiest to see. The ventral bundles lie deep, ventral to the gut, and are less easily seen.
There is a thin, inconspicuous layer of circular muscles outside the thick longitudinal bundles. It is not visible now.
There is also a thin connective tissue dermis, the simple epidermis, and the cuticle. These layers of the body wall will be more apparent in the cross sections which you will examine later.
The two black acicula of each parapodium can be seen protruding into the body cavity near the ventral edge of the dorsolateral longitudinal muscle bundle. Several small muscles originate in the body wall and insert on the proximal ends of the acicula. These muscles move the acicula, and as a consequence, the parapodium. There are other muscles inside the parapodium. The proximal ends of the acicula and the insertions of the muscles are covered by a cap of tissue, the chaetal sac, that secretes the chaetae and acicula.
A thick layer of oblique muscles extends from the ventral midline across the body to the ventral edge of the dorso-lateral muscle bundle (Fig 4). The circular muscles and ventral longitudinal muscles are hidden from view by oblique muscles. From your present vantage point its fibers appear to be oriented like those of circular muscles and, in fact, their long axes lie in the same plane (transverse plane) and both are perpendicular to the longitudinal fibers.
Notice the segmented condition of the coelom. Each segment has its own coelomic compartment separated (incompletely) from those of its neighbors by a transverse septum. Most of the septa were probably destroyed when you opened the coelom but remnants should still be present near the gut. The septa are perforated so the coelomic compartments are not completely isolated from each other.
Digestive System
The gut consists of an anterior, ectodermal foregut, a middle endodermal midgut, and a short, posterior, ectodermal hindgut. It is relatively simple and most of its length is the intestinewhere hydrolysis, absorption, and feces formation and storage occur. The intestine is the midgut.
The foregut is divided into a short anterior buccal cavity, a pharynx, and an esophagus. It extends from the mouth on the peristomium posteriorly to about segment 10. The buccal cavity is small and cannot be clearly discerned at present but you saw it earlier, turned inside out. The mouth opens into the buccal cavity.
The pharynx (Fig 3, 13-10A) is large and conspicuous. It has thick muscular walls and is invested with a tough connective tissue sheath. Numerous short pharyngeal protractor muscles run obliquely from the body wall to the anterior end of the pharynx. A few longer muscles run to the posterior end of the pharynx. Other muscles are confined to the pharynx and can be seen running longitudinally from its anterior end to its posterior.
The gut narrows posterior to the pharynx to become the esophagus (Fig 3, 13-10A). This is the posteriormost extent of the foregut. Two esophageal ceca lie free in the coelom and are diverticula of the esophagus at the point where it leaves the pharynx. These ceca are hollow and their lumina are continuous with the esophageal lumen. They secrete digestive enzymes as does the anterior region of the intestine.
Note that septa are absent from the anterior coelom in which the foregut lies. Remember that much of the foregut is everted during feeding, a feat that could hardly be accomplished if the foregut were anchored by septa. Notice that the anterior gut is little too long to fit into the anterior end of the worm without bending. This portion straightens when the pharynx is everted.
Posterior to the short esophagus is the midgut, or intestine, which extends almost the entire remaining length of the animal (Fig 3).
Several pharyngeal retractor muscles insert at the junction of the esophagus and intestine. These muscles originate on the midline of the dorsal body wall and are short when contracted. You may have cut them when you opened the body cavity. Contraction of the retractor muscles pulls the esophagus posteriorly and this in turn pulls the pharynx back into the coelom, thereby retracting it. The pharynx is protracted by contraction of the pharynx protractor muscles which insert on the anterior body wall and by increase in coelomic pressure generated by contraction of circular and oblique body wall muscles.
Note that the walls of the intestine are thinner than those of the foregut. Septa, which were absent from the region of the foregut, are present in the intestinal region with the exception of the first one or two segments, where the pharynx retractor muscles are located. The intestine empties into a short rectum (hindgut) in the last segment. The rectum empties to the exterior via the anus.
" With your fine scissors make an opening in the dorsal wall of the anterior intestine. Cut a middorsal longitudinal incision anteriorly from this opening along the entire length of the anterior midgut and foregut until you can see the mouth from inside the pharynx. Do not cut into the prostomium. Use a squeeze bottle or Pasteur pipet to squirt the gut contents out of the way.
Observe the morphology of the connection between the esophagus and the intestine. The esophagus narrows to form a small nozzle that protrudes into the anterior end of the intestine. This sphincter valve between the esophagus and intestine marks the division between foregut and midgut.
Compare the epithelial lining of the foregut with that of the midgut. It is easy to see the cuticle on the walls of the ectodermal foregut but there is none on the endodermal midgut. The inner surface of the esophagus is covered with large blunt papillae. The lumen of the pharynx is separated from that of the esophagus by a circular ridge of tissue. The walls of the pharynx are thick and muscular and its inner epithelium is covered by the thick glistening cuticle.
Find the opening from the anterior esophagus into one of the esophageal ceca. Verify that the cecum opens from the esophagus, not the pharynx, and then open the cecum to convince yourself that it is hollow.
Find the two large, black jaws protruding into the pharyngeal lumen from its sides. The region posterior to the jaws (postdental region) is never everted but everything anterior to it (predental region) is everted during feeding. The walls of the pharynx bear low, wide, longitudinal folds easily distinguished from the papillae of the esophagus.
Hard, black chitinous denticles can be seen on the walls of the predental pharynx. Anterior to the pharynx is the short buccal cavity. Its walls lack denticles.
Hemal System
The basic annelidan circulatory pattern is present in Nereis. It consists of dorsal and ventral longitudinal vessels connected to one another in each segment by segmental vessels to and from beds of narrow capillary-like vessels in the various organs (Fig 13-25A). The dorsal blood vessel, which you have seen already, is contractile and in it blood is moved anteriorly by peristalsis (Figs 3, 4).
Gently push the pharynx and anterior intestine aside to find the large median ventral blood vessel. Blood moves posteriorly in the ventral vessel and leaves it via afferent segmental vessels to the gut, body wall, nephridia, and parapodia. The respiratory surfaces are the dorsal ligules of the notopodia and they have a rich blood supply. Efferent segmental vessels drain these structures into the dorsal vessel. Anteriorly the dorsal vessel gives off two lateral vessels that run to a plexus of vessels in the walls of the pharynx and esophagus. These plexi drain into the ventral vessel.
Excretory System
Nereis has a pair of metanephridia in each segment except the first and last (Fig 13-10A). These are small, however, and very difficult to find. Lumbricus is a much better annelid in which to observe metanephridia. Nereis, a marine worm, is isosmotic to its environment whereas Lumbricus, a terrestrial worm, is hyperosmotic. How might this explain the relative sizes of their metanephridia?
A nephridium begins with a ciliated nephrostome located very low (ventral) on the anterior side of each septum (Fig 13-26D). The tubule from the nephrostome penetrates the septum to enter the coelom of the adjacent segment. Here it coils upon itself and forms a tiny knot, the convoluted nephridial canal, enclosed in a mesoepithelial sac. The tubule exits the sac and extends to the nephridiopore at the ventral base of the parapodium. The nephridia are difficult to demonstrate and are not visible without additional dissection.
>1b. As an optional exercise you may wish to expose a nephridium. The nephridia (Fig 13-10A) are hidden under layers of body wall muscles. They are best developed and easiest to see posterior to the pharyngeal region. Pick a segment in the vicinity of segment 20-25 that has not been damaged by your other activities. Push the gut aside and pin it out of the way. Look at the floor of the coelomic cavity and find the protruding ends of the acicula (do not mistake them for nephridia). They are covered by the soft, white, secretory chaetal sac (Fig 3) and have numerous small muscles attached to them. You found them earlier.
The metanephridium lies anterior to the acicula and under (ventral to) the oblique muscle at the lateral edge of the ventral longitudinal muscle. This part of the nephridium, which is the convoluted nephridial canal (Fig 13-26D), is a more or less pear-shaped mass deep below the oblique muscle of the coelom floor. It is entirely hidden from view by the oblique muscle and sometimes by the edge of the ventral longitudinal muscle. The tiny white nephrostome may be seen on the anterior side of the anterior septum of the segment. The nephrostome lies far ventral on the septum, close to the ventral body wall, and is associated with a blood vessel.
Remove the metanephridium, making an effort to get the nephrostome along with it. Prepare a wetmount and examine it with the compound microscope. The nephrostome is a funnel that bears long spikelike ciliated processes around its opening (Fig 13-26D). The cilia are long. The duct leading from the nephrostome is ciliated also. You will probably see the cilia beating if your specimen is alive but the heat of the substage lamp will soon kill the cells and immobilize the cilia. <
Nervous System
Nereis has a typical annelid nervous system (Fig 13-6A). First find the conspicuous, white, longitudinal, double ventral nerve cord lying on the ventral midline of the floor of the body cavity (Fig 4, 13-25A). The ventral blood vessel lies on top of it (dorsal) and partly obscures it. It is easiest to see in the pharyngeal region where both the gut and the blood vessel can be moved aside to give a clear view.
A swollen segmental ganglion is present on the nerve cord in the center of the floor of each segment. Three pairs of segmental nerves arise from most of the ganglia.
In the floor of the peristomium the ventral nerve cord dxpands to form the subpharyngeal ganglion and then bifurcates into right and left circumpharyngeal connectives that encircle the buccal cavity (Fig 13-14). These are easily seen by simply reflecting the anterior gut. The two connectives join each other dorsally at the brain in the prostomium. (There are, in fact, two connectives on each side.)
" Working under magnification, cut the pharyngeal protractor muscles on one side so you can better see the connectives. Trace one of them anteriorly, around the gut to the prostomium. Reflect and pin the gut as necessary. The connectives run to small peristomial ganglia beside the brain (Fig 13-14). The brain is large, bilobed, and white. It consists of two cerebral ganglia.
The brain is invested dorsally by tough connective tissue from which it is difficult to separate. You can see the ventral surface of the brain more easily than the dorsal. The brain occupies most of the posterior prostomium and the four eyes are embedded in it. Nerves from the sense organs of the head enter the brain.
Reproductive System
Nereis, like most polychaetes, is gonochoric, but lacks permanent gonads. During the reproductive season special areas of cells in the ventral peritoneum release immature gametes into the coelom. Here they complete their maturation and are eventually released into the sea by rupture of the body wall. In Nereis the nephridia are too small to function as coelomoducts for the release of gametes, especially ova. Thus your specimen has no gonads or gonoducts for you to find. On the other hand, depending on season, gametes may be present in the coelom. Living ova are a beautiful pale aqua color and may fill the coelom to such an extent that the entire worm is green.
If gametes are present, make a wetmount and examine it with the compound microscope. Ova are large nucleated spheres whereas sperm are tiny flagellated cells.
Many nereids transform into a special reproductive form called an epitoke, or heteronereid, modified for reproduction (Fig 13-30A). Well-developed epitokes have enhanced sensory and locomotory systems including enlarged eyes and paddle-like swimming setae. The epitoke of N. virens, however, is only slightly modified from the normal nonreproductive (atoke) condition. During the reproductive period in the spring and summer the epitokes leave the bottom and swarm at the surface. They rupture and release gametes into the sea and then die. Fertilization occurs in the sea and results in typical spiralian development and a trochophore larva.
Cross Section
Examine a commercially prepared cross section slide of Nereis and identify as many structures as possible (Fig 4, 13-6B).
The body is covered by an outer integument consisting of the extracellular, cuticle secreted by the underlying, monolayered epidermis. Inside the epidermis is the basal lamina and the connective tissue dermis.
A layer of circular muscle encircles the section just under the integument. The longitudinal muscles are divided into four conspicuous bundles; two dorsolateral muscle bundles and two ventrolateral muscle bundles. A layer of oblique muscles covers the ventrolateral muscles. Try to determine the orientation of the long axis of the muscle fibers in the muscle bundles. In a cross section the fibers would be cut in cross section in the longitudinal muscles and longitudinally in the circular and oblique muscles. Thus the muscle cells should look very different in the two types of muscles.
Figure 4. Cross section of Nereis virens. Poly79L.gif
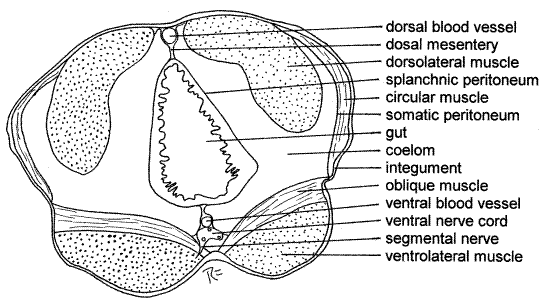
The innermost layer of the body wall is the somatic mesothelium, from which the muscles and peritoneum are derived. The cavity surrounded by the muscles is the coelom.
The gut is visible near the center of the section surrounded by the coelom (Fig 4). The gut wall consists of a monolayered mucosal epithelium, connective tissue layer, circular muscle, and splanchnic peritoneum, the latter lining the coelom. The gut wall encloses the gut lumen. Some of the peritoneum may be thickened to form specialized chlorogogen tissue.
The somatic and splanchnic peritonea are continuous with each other dorsal and ventral to the gut. Dorsally the connection forms the dorsal mesentery whereas ventrally it is the ventral mesentery. The mesenteries, like the septa, are double sheets of peritoneum.
The dorsal longitudinal blood vessel is in the dorsal mesentery, sandwiched between the two peritonea. The ventral longitudinal blood vessel and the ventral nerve cord are in the ventral mesentery. In addition, also depending on the plane of section, you may also see segmental ganglia, segmental nerves, metanephridia, acicula, and parapodia or parts of parapodia.
References
Brown FA . (ed) 1950. Selected Invertebrate Types. Wiley, New York. 597p.
Pettibone MH . 1963. Marine polychaete worms of the New England region. Bull. Mus. Nat. Hist. 227 :1-356.
Ruppert EE, Fox RS, Barnes RB. 2004. Invertebrate Zoology, A functional evolutionary approach, 7 th ed. Brooks Cole Thomson, Belmont CA. 963 pp.
Turnbull FM. 1876. On the anatomy and habits of Nereis virens. Trans. Connecticut Acad. Arts. Sci. 3:265-281.
Supplies
Dissecting microscope
Compound microscope
Long narrow dissecting pan such as can be made from kippered herring tins or aluminum ice trays (see Techniques chapter)
Living or preserved Nereis
Isotonic magnesium chloride for living worms
Dissecting set with microdissecting tools
# 1 stainless steel insect pins
cross section slide with parapodia