Invertebrate Anatomy OnLine
Mytilus edulus ©
Blue mussel
4jul2006
Copyright 2001 by
Richard Fox
Lander University
Preface
This is one of many exercises available from Invertebrate Anatomy OnLine , an Internet laboratory manual for courses in Invertebrate Zoology. Additional exercises can be accessed by clicking on the links to the left. A glossary and chapters on supplies and laboratory techniques are also available. Terminology and phylogeny used in these exercises correspond to usage in the Invertebrate Zoology textbook by Ruppert, Fox, and Barnes (2004). Hyphenated figure callouts refer to figures in the textbook. Callouts that are not hyphenated refer to figures embedded in the exercise. The glossary includes terms from this textbook as well as the laboratory exercises.
Systematics
MOLLUSCA P, Bivalvia C, Pteriomorphia sC, Isofilibranchia SO, Mytiloida O, Mytiloidea SF, Mytilidae F (Fig 12-122, 12-125)
Mollusca P
Mollusca, the second largest metazoan taxon, consists of Aplacophora, Polyplacophora, Monoplacophora, Gastropoda, Cephalopoda, Bivalvia, and Scaphopoda. The typical mollusc has a calcareous shell, muscular foot, head with mouth and sense organs, and a visceral mass containing most of the gut, the heart, gonads, and kidney. Dorsally the body wall is the mantle and a fold of this body wall forms and encloses that all important molluscan chamber, the mantle cavity. The mantle cavity is filled with water or air and in it are located the gill(s), anus, nephridiopore(s) and gonopore(s). The coelom is reduced to small spaces including the pericardial cavity containing the heart and the gonocoel containing the gonad.
The well-developed hemal system consists of the heart and vessels leading to a spacious hemocoel in which most of the viscera are located. The kidneys are large metanephridia. The central nervous system is cephalized and tetraneurous. There is a tendency to concentrate ganglia in the circumenteric nerve ring from which arise four major longitudinal nerve cords.
Molluscs may be either gonochoric or hermaphroditic. Spiral cleavage produces a veliger larva in many taxa unless it is suppressed in favor of direct development or another larva. Molluscs arose in the sea and most remain there but molluscs have also colonized freshwater and terrestrial habitats.
Eumollusca
Eumollusca, the sister taxon of Aplacophora, includes all molluscs other than aplacophorans. The eumolluscan gut has digestive ceca which are lacking in aplacophorans, the gut is coiled, and a complex radular musculature is present.
Conchifera
Conchifera, the sister taxon of Polyplacophora, includes all Recent molluscs other than aplacophorans and chitons. The conchiferan shell consists of an outer proteinaceous periostracum underlain by calcareous layers and is a single piece (although in some it may appear to be divided into two valves). The mantle margins are divided into three folds.
Ganglioneura
Most Recent molluscs are ganglioneurans, only the small taxa Aplacophora, Polyplacophora, and Monoplacophora are excluded. Neuron cell bodies are localized in ganglia.
Ancyropoda
The mantle cavity, with its gills, is lateral. The calcareous portion of the shell is bivalve, with the valves opening laterally and joined dorsally by a derivative of the periostracum.
Bivalvia C
Bivalvia is a large, successful, and derived taxon. The body is laterally compressed and enclosed in a bivalve shell. The two valves are hinged dorsally. The the foot is large and adapted for digging in the ancestral condition. A crystalline style is usually present but never is there a radula. The mantle cavity is lateral and in most bivalves the gills are large and function in respiration and filter-feeding. The head is reduced and bears no special sense organs. The nervous system is not cephalized. The group includes scallops, clams, shipworms, coquinas, marine and freshwater mussels, oysters, cockles, zebra mussels, and many, many more.
Metabranchia sC
Most bivalves are metabranchs. The gills are adapted for filter feeding and water enters the mantle cavity posteriorly.
Filibranchia SO
Filibranchs are suspension-feeding bivalves with filibranch gills.
Pteriomorpha O
Pteriomorph bivalves are epibenthic and live on, rather than in, the bottom. They may be attached or unattached, may have a byssus or not, and may cement one valve to the substratum or not. The foot is reduced and the mantle margins are not fused. The gills are large and used for filter feeding. There is a tendency to reduce or loose the anterior adductor muscle. Siphons are absent or reduced. This taxon includes the well-known arcs, mussels, scallops, pen clams, and oysters.
Laboratory Specimens
Marine mussels are metabranch bivalves with filibranch gills. Almost any marine mussel will serve for this exercise as most are similar in the basic features of their anatomy. The exercise is written specifically for Mytilus edulis, the blue mussel (Fig 12-110A), with parenthetical comments on differences from Geukensia demissa, the ribbed mussel (Fig 12-110E). From the standpoint of general anatomy the differences between these two species are minor. Both are common but Mytilus is more widespread and has the added advantage of being available alive in inland fish and supermarkets. Freshwater mussels are unrelated and are not described by this exercise.
Mytilus edulis is found, often in high population densities, in Europe, on both coasts of North America from the Arctic to the Middle Atlantic States on the east coast and south to California on the west. It forms dense and extensive mats on hard substrata. It is a valued seafood and supports a commercial fishery where it is abundant. It reaches about 8 cm in length. Large specimens are often overgrown by other organisms.
Geukensia demissa occurs along the east coast of North America from Nova Scotia to Florida and is common in salt marshes and among oysters in the southeastern United States. It is an intertidal species reaching its largest size (about 10 cm) in salt marshes where it lives in the sediment attached to saltmarsh cord grass, Spartina alterniflora (Fig 12-110E).
Living specimens should be relaxed in isotonic magnesium chloride and dissected under magnification, immersed in magnesium chloride in a small dissecting pan.
Shell
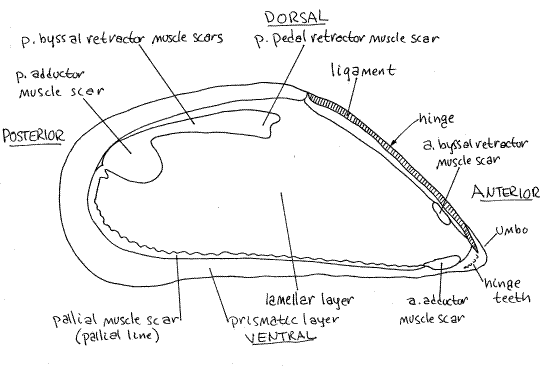
Obtain an empty shell for study. Like that of all bivalves, it is composed of two similar valves. Each valve is elongate and the exterior is relatively smooth but has concentric growth rings. (The valves of Geukensia are sculptured with strong radial ribs). The anterior end is pointed and the posterior is broadly rounded (Fig 1). The dorsal margin is convex whereas the ventral is weakly concave. There is a slight permanent gape, where the valve margins do not meet, near the middle of the ventral midline between the two valves. This is the byssal gapewhich accommodates the byssus.
The two valves are held together in life by the long, straight, developed hinge occupying the anterior end of the dorsal margin (Fig 1). Note the narrow, straight, proteinaceous ligamentextending for the length of the hinge. Hinge teeth are weakly developed in mussels. The inconspicuous umbos are located at the anterior end in Mytilus (but are a little posterior to the end inGeukensia). The valve is covered by the conspicuous, dark yellowish brown or black, proteinaceous periostracum. This outermost layer of the shell can be seen folded over the ventral edge of the valve in fresh specimens. The three shell layers are, from outside in, the organic periostracum, calcareous prismatic layer (= ostracum), and calcareous, nacreous, lamellar layer (= hypostracum) (Fig 12-91). The valves are often eroded so that the chalky white calcareous prismatic layer shows through the dark periostracum
Use your knowledge of the antero-posterior and dorso-ventral axes to determine which valve is right and which left. Mussels are equivalve (right and left valves are nearly identical and symmetrical). The opposite is inequivalve. Look at the outside of one of the valves. Each valve is strongly asymmetrical, a condition referred to as inequilateral. The anterior end of the valve does not resemble the posterior (Fig 1). The opposite is equilateral.
Most of the interior of the valve is pale but the margins are dark. The line separating the two is the pallial line (Fig 1). It is the line of attachment of the mantle to the valve.
A small anterior adductor muscle scar can be seen at the anterior end of the valve (Fig 1). It lies on the pallial line on the ventral edge of the valve. The much larger posterior adductor muscle scar is located at the posterior end, displaced to the dorsal side. These scars mark the sites of attachment of the adductor muscles to the valves. Remember their location.
The scar of the anterior pedal-byssal retractor muscle is a small, pale, slender, elongate depression under the overhang of the anterior edge of the valve below the ligament (Fig 1). The scar of the posterior pedal-byssal retractor muscle is a large, lobed, narrow, dark area extending anteriorly from the dorsal edge of the posterior adductor scar.
Figure 1. Interior of the left valve of Mytilus edulis (redrawn from White, 1937). Bivalve70La.gif
External Anatomy
" Living (or preserved) Mytilus are easily opened, unlike Mercenaria). Hold the mussel with its right valve uppermost. Insert the tip of a screwdriver or the blunt end of a forceps into the byssal gape and twist it to force the valves to gape all around, especially posteriorly. Refer to an empty valve to help you remember the position of the posterior adductor muscle. This muscle is located near the dorsal margin just posterior to the hinge.
Slip a long, sharp scalpel blade into the posterior gape and cut the posterior adductor muscle. The muscle is easily recognized by feel because it is the only firm, resisting structure in the vicinity. You can also look into the gape and see it. Try to avoid cutting any other tissues around the muscle. It will, however, be necessary to cut the black tissue connecting the right and left mantle skirts at the siphons. The rectum and anus are also in this vicinity and you will want them intact later.
Cut the small anterior adductor muscle. The anterior adductor is on the ventral margin just posterior to the anterior tip of the shell.
Lift the right valve slightly and separate it from the soft tissue (right mantle skirt) adhering to its inside surface. Use a scalpel to scrape the right posterior pedal-byssal retractor muscle and the right anterior pedal/byssal retractor muscle away from the right valve.
Remove the right valve. The well developed proteinaceous ligament that extends along most of the length of the hinge (Fig 1, 3) and must be cut or torn to remove the valve. Place the left valve, with the animal contained in its concavity, in a 12 cm culture dish or small dissecting pan of isotonic magnesium chloride (use tapwater for preserved specimens).
Later in the exercise, after the animal has relaxed, replace the magnesium chloride with seawater so the heart will continue beating, or resume beating if it has stopped. The animal must be immersed in fluid during the dissection and most of the work should be done on the stage of the dissecting microscope.
Figure 2. Lateral view of a dissected Mytilus edulis from the right side (redrawn from White, 1937). The right valve, mantle skirt, gill, and labial palps have been removed. Part of the right gill has been removed. The visceral mass has been opened and much of it has been removed. Bivalve71La.gif
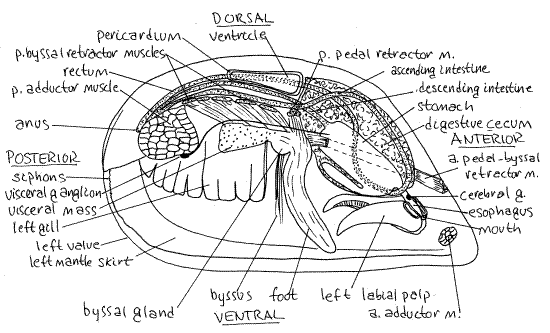
Mantle
The animal is enclosed by the large right and left mantle skirts (lobes) which line the inner surfaces of the two valves (Fig 2). The space between the two mantle skirts is the inhalant chamber of the mantle cavity (Fig 3). In life it is filled with seawater. The two skirts are connected dorsally to each other and are attached to the valves along the pallial line.
The mantle skirts are much thicker than is usual in bivalves because they contain the gonads (Fig 3). When ripe, the male mantle is creamy beige whereas that of females is reddish.
Figure 3. Cross section of Mytilus edulis (redrawn from White, 1937). The section is at a level immediately posterior to the foot. A posterior curve of the distal foot resulted in its inclusion in this section. o = outer, m = middle, i = inner. Bivalve72La.gif
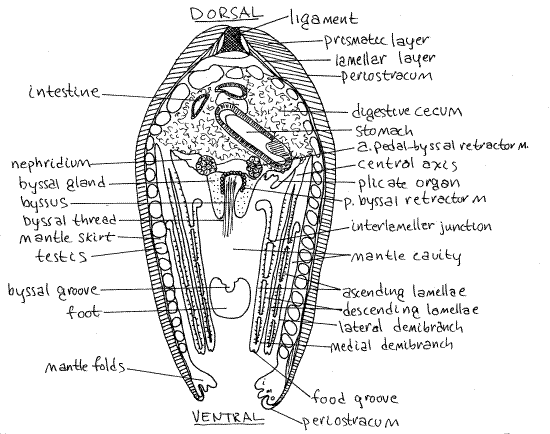
Find the left mantle skirt. It should still be attached to its valve. The mantle margins of mussels provide a good example of the basic tripartite condition characteristic of most bivalves. Look at the ventral margin of the mantle skirt. This margin should still be attached to the margin of the left valve. It consists of three folds; outer, middle, and inner (Fig 3). The muscular inner fold is medial and is the largest. It forms a conspicuous bulging ridge running along the edge of the mantle margin and it bears a thin papillate crest extending along its length.
Lateral to the inner fold is the much smaller, sensory middle fold. The periostracal groove separates the middle fold from the outer fold. The periostracum is secreted by the inner (medial) surface of the outer fold and thus originates in the periostracal groove. The outer fold is secretory but cannot be seen clearly because it is covered by the periostracum, which it secreted.
The newly secreted periostracum remains attached to the bottom of the periostracal groove (Fig 3) and extends outward from the groove, over the outer fold, and around the edge of the valve. Grasp the newly formed periostracum with a fine forceps and tug it. It is very tough and pliable. Tear a bit of it away so you can see the outer mantle fold. The outer surface of the outer fold secretes the prismatic (middle) layer of the shell. The entire outer surface of the mantle skirt (not fold) secretes the lamellar (inner) layer and is in broad contact with it.
" Cut away the newly secreted periostracum on the edge of the left valve along a 2 cm length of the valve margin. Use fine scissors or a scalpel to do this. Lift the freed mantle margin and look beneath it at the shell. You will see that the mantle is attached to the shell a short distance from its margin. This attachment is accomplished by the pallial muscles in the muscular fold (inner fold) of the mantle margin. This line of muscle attachment parallels the margin of the valve and is the pallial line. Posteriorly, the right and left mantle skirts together form the obscure ventral inhalant and dorsal exhalant siphons (Fig 2). The skirts are connected by a small, transverse, dark brown branchial membrane between the two siphons. The epithelium in the vicinity of the siphons is darkly pigmented. A vertical, median membrane extends ventrally from the branchial membrane. Neither of the two siphons is distinct. They are weakly modified areas of the mantle margin and are not complete tubes as are the siphons of many bivalves. They are short and do not extend from the shell. Being epifaunal, Mytilus does not need the tubular siphons characteristic of infaunal burrowers.
Adductor Muscles
Examine the two adductor muscles which you cut in order to open the animal (Fig 2). The anterior adductor muscle is reduced and is much smaller than the posterior adductor muscle as it is in all mussels. This disparity in the size of the two adductor muscles is referred to as the heteromyarian condition and it is associated with the presence of a proteinaceous holdfast called the byssus, which will be discussed later. The heteromyarian condition is derived from the more primitive, and more common, dimyarian condition in which the two adductor muscles are of similar sizes.
Gills
" Use scissors to remove the right skirt of the mantle. This will expose the large right gill extending the length of the mantle cavity on the right side of the visceral mass (Figs 2, 3, 12-110B). A gill is formed of the combined filaments attached to the central axis. The entire gill is a holobranch (=whole gill) and includes the filaments on both sides of the axis. There is only one gill on the right and one on the left even though it may look to you as if there are two on each side.
Each holobranch consists of two demibranchs, or half gills, one medial and one lateral (Fig 3, 12-96). Do not mistake demibranchs for complete gills. Find the lateral and medial demibranchs of the right gill.
Each of the two surfaces of a demibranch is a lamella. Each demibranch thus has two lamellae (Fig 3, 12-100). Each holobranch, since it is composed of two demibranchs, has four lamellae.
The two demibranchs are attached to each other along the central axis (Fig 3, 12-96). This longitudinal axis extends the length of the dorsal wall of the mantle cavity between the visceral mass and the mantle skirt. The demibranchs hang freely into the mantle cavity and are attached to the body wall at the central axis.
In cross section each holobranch can be likened to a capital W (\/\/) or, more accurately, a "double V" (Fig 3, 2-96C,D). The central axis by which the gill is attached to the body is represented by the middle point of the W. Each V of the \/\/ is a demibranch. Each demibranch (V) is composed of two lines, \ and /, which represent the lamellae. The four lamellae of the holobranch are the four straight lines of which the W is composed (i.e. \ / \ /).
The two lamellae that drop from the central axis down into the mantle cavity are the descending lamellae (Fig 3, 12-100). Each holobranch has two, each demibranch has one. The descending lamellae of adjacent demibranchs face each other. The lamellae that rise back up into the mantle cavity are the ascending lamellae. The descending lamellae of a holobranch are the inner two straight lines of our W (/\) and the ascending lamellae are the outer two (\ /).
In mussels and scallops, the upper ends of the ascending lamellae are not attached to the body wall and are entirely free (Fig 3, 12-100). The ascending lamella of the lateral demibranch of the right gill is facing you now (if you have been following instructions). Put your new knowledge to use by naming the remaining three lamellae of the right gill.
Look at the surface of a lamella under magnification and see the numerous very narrow gill filaments of which it is composed. They are not grouped into ridges (plicae) as are those of most bivalves, and the surface of the demibranch is flat, except for the minute ridges of the individual filaments.
> 1a. If your animal is alive, you can see cilia beating on the surface of the gills. This is best accomplished by removing the animal from its dish and looking at the gills, with magnification, while they are covered by a thin film of water. Look at places where light is reflected from the surface and you will see it shimmer from the activity of the cilia. <
In mussels, most ciliary currents on the lamella beat ventrally toward the ventral food grooves on the ventral to edges of the demibranchs (Fig 3, 12-99A). In mussels the dorsally directed currents are weak. The currents in the ventral food grooves are longitudinal, toward the labial palps at the anterior end. Look carefully at the ventral edge of a demibranch and find its food groove. A weak anterior longitudinal current exists along the dorsal free edge of the ascending lamellae but not along the central axis of the holobranch.
>1b. If you have a living mussel, place it in a dish of seawater and arrange it in the dish so the flat surface of the exposed lamella is horizontal, or nearly so. Look at the surface of the gill with magnification. Place a little volcanic ash, chalk dust, or a drop of carmine-seawater suspension on the surface of the gill while watching it with the dissecting microscope. You should be able to see the particles being moved quickly over the gill to the longitudinal food groove on its ventral margin. The particles, and the mucus surrounding them, are moved anteriorly by the ciliary transport mechanism of the food groove. Leave the mussel in seawater so its heart will recover and resume beating. <
Mussels and scallops have filibranch gills. Such primitive and are presumed to be the original condition of the lamellibranch gill. In a filibranch gill, adjacent filaments are held together only by ciliary interfilamentar junctions and are easily pulled apart (Fig 12-98A,B). The eulamellibranch gills of most other bivalves, such as Mercenaria and Corbicula, are held together by solid, vascularized tissue junctions (Fig 12-98C,D). The filaments of eulamellibranch gills have, in fact, grown together to form a continuous sheet perforated by small pores.
Each filament bears frontal cilia on its outer edge and lateral cilia on the flat surfaces facing adjacent filaments (Fig 12-97B, 12-98B). The lateral cilia generate the feeding/respiratory current whereas the frontal cilia move food particles along the surface of the gill to the food grooves.
>1c. If you have a living specimen, you can demonstrate the roles of the frontal and lateral cilia. Locate the free dorsal edge of the ascending lamella of the lateral right demibranch. That is the lamella facing you. While watching under magnification, place a drop of dye/seawater solution on the surface of the lamella. Some of the dye will be propelled by the frontal cilia toward the ventral food groove but some of it will be moved through the gill and into the exhalant chamber by the lateral cilia. Because the dorsal edge of the lamella is unattached, you can see the interior of the exhalant chamber and can see the current of dye appear suddenly in this space. <
The gills divide the mantle cavity into a ventral inhalant chamber and a dorsal exhalant chamber. Water enters the inhalant chamber from the ventral inhalant siphon (Fig 12-89A). It then passes between the gill filaments to enter the exhalant chamber. It flows back into the sea through the dorsal exhalant siphon. The large inhalant chamber is readily visible between the two mantle skirts whereas the exhalant chamber can be seen only by removing or opening a demibranch.
Use a minuten nadel to separate two adjacent gill filaments from each other and then look inside the demibranch. Note that there are no tissue connections between adjacent filaments and thus they are easily separated. The space you now see within the demibranch is the exhalant chamber. The chamber is not divided into water tubes or vertical channels as it is inMercenaria and Argopecten. Note that adjacent filaments are held together loosely by cilia and not by permanent tissue junctions. This is the defining characteristic of filibranch gills.
>1d. Remove about 2-3 mm of the edge of a demibranch, place it on a slide, tease the filaments apart, affix a coverslip, and examine it with the compound microscope. If your specimen is alive, the beating cilia of the filament will be easy to see. Look for the ventral food groove at the edge of the gill. Note that it is a deep groove with a narrowed opening formed by the ends of the filaments of the descending and ascending filaments. In living specimens, the beating cilia of the filaments are easily seen. See if you can distinguish the lateral from the frontal cilia. <
Adjacent lamellae (not filaments) are held together by widely spaced interlamellar junctions (Fig 3, 12-96). These are small "spot welds" and are not continuous and do not divide the exhalant chamber into water tubes. Pull the dorsal edge of the ascending lamella of the lateral demibranch toward you so you can see into the exhalant chamber. There you will see numerous interlamellar junctions holding the ascending and descending lamellae together.
Any kind of junction between opposing lamellae is an interlamellar junction. Any junction between adjacent filaments is an interfilamentar junction. The interfilamentar junctions of mussels are ciliary whereas the interlamellar junctions are small tissue connections between the descending and ascending lamellae of a demibranch.
Labial Palps
At the anterior end of the visceral mass is a pair of elongate, triangular, flat labial palps, one right and one left (Fig 2, 12-89A, 12-100). The palps are ciliated and are used to transfer food from the gills to the mouth. Each palp consists of a lateral and a medial lamella associated with the lateral and medial demibranchs respectively. There is one lamella for each demibranch. One surface of each lamella is covered by ciliated ridges and grooves. The ridged surfaces of the two lamellae of a palp face each other. The ridges are perpendicular to the long axis of the palp. A longitudinal ciliated oral groove extends along the junction between the two lamellae.
Each lamella, medial and lateral, is connected physically with its counterpart on the opposite side. These transverse connections form a pair of lips above and below the mouth (Fig 12-100). Thus the right and left lateral palps are connected with each other by the dorsal lip above the mouth and the right and left medial palps are connected by the ventral lip below the mouth.
The mouth is a small opening located on the anterior midline of the visceral mass between the dorsal and ventral to lips. The oral groove runs between the upper and lower lips to enter the mouth.
The ciliated ridges and grooves form a sorting field to partially separate the mineral and organic particles collected on the gill surfaces. Ciliary currents in the grooves move mineral particles to rejection currents along the free margins of the lamella. Ciliary currents on the crests of the ridges and in the oral groove move organic particles toward the mouth. The oral groove transports these particles, between the lips, to the mouth. Final sorting will occur in the stomach.
The rejection current runs along the free edge of the lamella to the pointed tip of the palp. Rejected sediment, trapped in mucus and known as pseudofeces, drops into the inhalant chamber of the mantle cavity. Posteriorly directed currents on the ventral mantle margin and posteroventral currents on the mantle surface move the pseudofeces posteriorly to the inhalantsiphon. The particles are ejected from this siphon when periodic contractions of the adductor muscles force spurts of water out of the inhalant chamber.
>1d. Place a little carmine-seawater on the opposing surfaces of the two left palps and watch it as it is transported by their cilia. Try to trace currents and watch for the development of a stream of particles in the oral groove leading into the mouth. This is probably the easiest way to find the mouth. The mouth is a small and inconspicuous pore but is easy to see if it has a string of red carmine particles entering it. <
Respiratory Surfaces
The gills are not the only, or even the most important, respiratory organs of mussels. The inner surfaces of the mantle skirts are also responsible for gas exchange but the chief respiratory surfaces are the plicate organs (Fig 3). Each of the two plicate organs is a longitudinal row of transverse folds of epithelium between the gill and the visceral mass. They are heavily vascularized, more so than the gills.
" Carefully remove the right gill to expose the right plicate organ. Look deep in the crease between the central axis of the gill and the visceral mass. In living specimens the folds are white and show up clearly against the golden brown kidney or dark brown digestive cecum behind them.
Visceral Mass
Removal of the right gill exposes the visceral mass and foot. You should now be looking at the exposed right side of the visceral mass (Fig 2). It is the largest part of the body and contains most of the organs. It lies on the median plane in the center of the mantle cavity. To the left of the visceral mass are the left gill, left mantle skirt, and left valve in that order. Point the anterior end of the animal to your right.
Look at the visceral mass lying between you and the left gill. It is elongated along its antero-posterior axis and is compressed from side to side. Look through the body wall for the large, dark brown digestive cecum, which occupies most of the space in the anterior visceral mass. It is located immediately posterior to the mouth and can be seen through the body wall. The esophagus, stomach, and much of the intestine are embedded in the digestive cecum and visceral mass but will not be seen at this time. If the right gill has been removed, the digestive cecum will be easy to see.
The nephridium is visible in the dorsal mantle cavity under the surface of the visceral mass beside the gill. It is yellow brown. The plicate organ is on its surface.
Foot and Byssus
The small wormlike foot is located in about the middle of the ventral margin of the visceral mass (Fig 2, 12-110B). In mussels the foot is not used for digging and is not a typical bivalve foot. Its function is the formation and manipulation of byssal threads.
The foot is muscular is composed of an outer layer of circular muscles around an inner core of longitudinal muscles. There are also some oblique fibers. The pedal hemocoel inside the foot is a hydrostatic skeleton that allows these muscles to antagonize each other. Contraction of the circular muscles extends the foot whereas contraction of the longitudinals withdraws it. Partial and selective contraction of the longitudinal muscles can bend the extended foot.
The foot has a longitudinal byssal groove along its posterior margin (Fig 3, 12-110B). The byssal gland is located at the base of the foot and byssal groove (Figs 2, 3, 12-110B). You may be able to see byssal threads emerging from it. The byssal groove originates at the gland, which secretes liquid protein that is formed into threads by the byssal groove. The threads are attached to the substratum by the foot and then allowed to harden.
Collectively the resulting cluster of protein threads is the byssus (Fig 2, 12-110B). It may or may not still be present in your animal. It is a holdfast extending from the visceral mass to the substratum. The proximal ends of the threads enter the visceral mass and are attached to numerous muscles, the byssal retractor muscles that run through the mass to insert on various parts of the shell (Fig 2). These white muscles can be seen easily through the integument of the visceral mass of Mytilus. (They are less easily seen in Geukensia.)
Pedal and Byssal Muscles
The foot and byssus are moved with respect to the body by a set of extrinsic muscles that originate on the shell and insert on the foot or byssal gland. Among these is a pair of anterior pedal-byssal retractor muscles of the foot and byssus (Fig 2). These are white, cordlike muscles extending from their origin at the base of the foot along the anterior edge of the visceral mass. They diverge slightly to their separate insertions on the shell under the anterior end of the ligament. You found their scars earlier and may want to refer once again to the empty valve to confirm their position.
Several pairs of posterior byssal retractor muscles originate at the base of the foot and fan out dorso-posteriorly to their insertions on the shell in a line anterior to the posterior adductor muscle. These are also white, cordlike paired muscles. The retractor muscles can retract the foot but their primary function is to pull the animal to the substratum to which the byssus is attached.
The anteriormost of this series of muscles is sometimes known as the posterior pedal retractor muscle (Fig 2).
Internal Anatomy
Hemal System
The pericardial cavity is located dorsally at the very top of the visceral mass, just ventral to the middle of the dorsal margin of the shell. In Geukensia it is ventral to the posterior third of the hinge and in Mytilus it is posterior to the hinge. It is a coelomic space and is lined with peritoneum. The walls of the cavity are relatively thick and opaque in Geukensia but thin and translucent in Mytilus.
" Open the pericardial cavity carefully with a shallow, dorsal, longitudinal cut made with fine scissors. It may already have been opened accidentally. The posterior intestine, which is the rectum, extends longitudinally through the cavity but is enclosed for most of its length by the ventricle of the heart.
The heart is associated with the rectum in the anterior part of the pericardial cavity. In Mytilus the single ventricle is a pale yellowish mass that surrounds the tubular rectum. At its anterior end the ventricle swells to form the aortic bulb at the base of the aorta. The rectum passes through the center of the bulb.
" With fine scissors open the left wall of the aortic bulb and look for the openings of the major arteries. The aorta is median and unpaired and exits the bulb on its dorsal anterior wall, dorsal to the rectum. It supplies the anterior mantle and dorsal esophagus with blood. The paired posterior pallial arteries arise from the lateral floor of the aortic bulb. They supply the posterior mantle. A very large, unpaired coeliac artery exits the floor of the aortic bulb on its midline. It immediately branches into several important arteries to the stomach, intestine, and digestive ceca. (In Geukensia, a posterior aorta exits the ventricle posteriorly and runs ventral to the rectum. There is no posterior aorta in Mytilus).
On each side the ventricle connects with a membranous atrium. Hold the mussel erect, with its dorsal edge up and focus on the ventricle. Pull the ventricle to one side and you will see the atrium being stretched on the opposite side by its connection with the ventricle. The atria extend ventrolaterally on the sides and floor of the pericardial cavity. Each of the two atria occupies most of the length of the cavity and is attached posteriorly to the other atrium. Their lumina are not continuous, however. The atria receive oxygenated blood from the efferent branchial vessels draining the gills.
The lobulated brown pericardial glands are associated with the atria. They are a part of the excretory system and are involved in the production of the primary urine in the pericardial cavity.
Reproductive System
Mussels are gonochoric. The gonads extend throughout most parts of the body except the gills, muscles, and foot (Fig 3). Most of the gonad is in the mantle skirts, thus accounting for the unusual thickness of the mantle in these bivalves. Some of the gonad is in the visceral mass (Fig 3). Ovaries are reddish in Mytilus, purplish in Geukensia whereas testes are cream inMytilus and yellow in Geukensia.
>1e. Make a smear preparation of a bit of the mantle of a mature specimen and examine it with the compound microscope for eggs or sperm. If your specimen is living, make the slide with seawater and look for motile sperm. <
On each side of the body the many lobes of the gonad connect by a converging system of ducts leading to the gonopore on the tip of the genital papilla. This papilla is located on the roof of the mantle cavity where the medial demibranch attaches to the visceral mass. This places it in the exhalant chamber dorsal to the medial demibranch but you can see it without dissection because the gill is not attached to the foot
Excretory System
The two nephridia, or kidneys, are complex, elongate, brown organs lying in the roof of the mantle cavity at the base of the gills (Fig 3, 12-118). They extend from the labial palps to the posterior adductor muscle. In your dissection the right kidney should be easy to see under the cut edge of the left gill and plicate organ. Anteriorly it may lie on the surface of the digestive cecum, which is also brown, but darker, so the two are readily distinguishable.
Each nephridium connects to the pericardial cavity via a renopericardial canal. Each of the two renopericardial canals opens from the pericardial cavity via a small pore in its anterior floor. Each nephridium empties into the exhalant chamber via a nephridiopore located atop the tiny urinary papilla on the base of the posterior side of the larger genital papilla.
Pericardial glands are also associated with the excretory system. The pericardial glands invest the outer walls of the atria and give that part of the heart its characteristic brown color.
Nervous System
The nervous system consists of the usual bivalve ganglia, connectives, and nerves. The major features of the system are superficial and can be revealed simply by removing the epidermis covering them. An extensive dissection is not necessary. Care should be taken to avoid damage to the digestive system, which you have not yet studied.
The paired cerebral ganglia are situated beside the esophagus (Fig 2). They are beside the posterior margin of the lower lip touching the medial edge of the anterior pedal-byssal retractor muscle. In life, the ganglia usually contain orange or reddish neuroglobin and, when they do, are easily seen through the thin integument, without dissection or magnification. If they lack this pigment (as preserved specimens always do) they will be harder to find.
" Carefully remove the integument covering the area described above. Look for the right cerebral ganglion in the corner between the lower lip and the anterior pedal-byssal retractor muscle. You may want to insert a blunt probe into the mouth to help you find the esophagus. The thick cerebral commissure arches over the esophagus to connect the right and left cerebral ganglia.
A large common trunk of the cerebropleural and cerebrovisceral connectives exits each cerebral ganglion and extends posteriorly beginning on the ventral border of the anterior pedal-byssal retractor muscle. The trunk curves around the lateral border of the retractor and ends on the dorsal surface of the posterior end of that muscle. On the way around the lateral edge of the muscle it bifurcates into the cerebropedal and cerebrovisceral connectives.
The cerebropedal connective extends posteroventrally along the anterior pedal-byssal retractor muscle to the pedal ganglion. The connectives and the ganglion can be seen by cutting the epithelium covering the groove between the pedal-byssal retractor and the visceral mass medial to it. The ganglion is usually yellowish or orange. Nerves to the foot, byssal gland, and both pedal-byssal retractor muscles arise from it. The two pedal ganglia touch each other on the median plane and their neurons are connected by the short transverse pedal commissure.
The cerebral ganglia, cerebral commissure, cerebropedal connectives, pedal ganglia, and pedal commissure make up a circumesophageal nerve ring that loosely encircles the esophagus. A small nerve arises at the point where the common trunk bifurcates. It runs to a statocyst located near the surface of the body in the angle between the cerebropedal and cerebrovisceral connectives.
The cerebrovisceral connnective on each side extends posteriorly across the outer surface of the digestive cecum in the visceral mass. It lies just inside the integument and crosses the lateral surfaces of the dorsal ends of the posterior pedal-byssal retractor muscle. It parallels and is medial to the line of attachment of the gill to the body. In this area it can be seen as a white band through the transparent epithelium. The cerebrovisceral connective has branches to the digestive cecum, intestine, gonad, and nephridia. The areas surrounding the retractor and adductor muscles may have been damaged when you cut the muscles to open the shell. If that is the case it may be necessary to trace the cerebrovisceral connective on the other side.
The cerebrovisceral connective extends posteriorly to the visceral ganglion (Fig 2) located on the ventral, slightly anterior, surface of the posterior adductor muscle. The right and left visceral ganglia are close together and are connected by the transverse visceral commissure. Nerves to the mantle, siphons, nephridia, foot, and posterior adductor muscle arise from the visceral ganglia. It is possible that the visceral ganglia were destroyed when you cut the posterior adductor muscle.
Digestive System
" Relocate the tiny slit-shaped mouth on the median plane between the upper and lower lips (Fig 2). It opens into a short, straight esophagus. Insert a blunt probe into the mouth to reveal the location of the esophagus. Leave the probe in the esophagus and with fine scissors cut along the probe. This will open the esophagus with a lateral longitudinal incision along the right side of the visceral mass. The esophagus runs straight posteriorly, angling slightly dorsally, to open into the anterior end of the stomach (Fig 2). The esophagus and stomach are embedded in the digestive cecum. Extend the incision posteriorly into the stomach.
" The esophagus widens suddenly in the anterior dorsal visceral mass to become the stomach located ventral to the posterior part of the hinge (Fig 2). Continue the incision along the left wall of the stomach. Rinse the inside of the stomach with squirts of water from a pipet and look at its lumen and walls.
The sorting field is a complex of ciliated ridges and grooves on the stomach wall whose function is to separate digestible organic particles from indigestible mineral particles (Fig 12-102). The organics are sent to the digestive cecum for digestion and the minerals directly to the intestine to become feces.
A thin chitinous plate, the gastric shield, is located on the anterior wall of the stomach. The crystalline style is a transparent, jelly-like rod that protrudes into the stomach from the style sac and rests against the gastric shield. It is composed of digestive enzymes, especially carbohydrases and lipases, but there are no proteases in it. The style is very long and extends far back into the style sac, which is combined side by side with the intestine (Fig 12-103A), to the level of the posterior adductor muscle. This portion of the intestine is known as the descending intestine (= direct intestine). You will follow it soon but in the meantime do not pull the crystalline style out of the style sac. The enzymes of the style are secreted by the style sac epithelium and the style can be reabsorbed when it is not needed. It is often absent in individuals that have not fed recently.
The style sac and descending intestine exit the posterior end of the stomach. They are joined side by side and their lumina are continuous. A pair of opposing typhlosoles, or ridges, separate the lumen of the tube into two parallel and contiguous channels, one of which houses the style and is the style sac. The other is the descending intestine. The style sac continues a distance posteriorly and then ends blindly.
Cilia in the walls of the style sac rotate the style causing its anterior end to rub against the gastric shield. This abrades its anterior end and releases enzymes into the stomach lumen. Rotation of the style also helps pull a string of mucus and food particles into the stomach from the mouth. This string originated on the gills and labial palps. Several ducts from the digestive cecum open into the stomach. Their number and position vary from individual to individual. In some individuals there is a ventral diverticulum of the stomach (Fig 2).
The intestine leaves the posterior end of the stomach and loops through the visceral mass before eventually reaching the anus. It is composed of three regions of approximately equal length (Fig 2), of which the last is the rectum. The rectum (Fig 2), which is the posterior third of the intestine, can be seen without any additional dissection as it passes through the pericardial cavity. The rectum ends at the anus located in the exhalant chamber above the posterior adductor muscle (Fig 2). The posterior rectum is often damaged when the posterior adductor muscle is cut to open the shell early in the dissection.
Although it is impractical to trace the intestine posterior to the style sac. The descending intestine extends posteriorly from the stomach and lies beside the style sac as has been discussed. The descending intestine and style sac continue side by side posteriorly to a position dorsal to the posterior adductor muscle. Here the intestine makes a sharp bend dorsally, to the right, and then turns and runs back anteriorly as the ascending intestine (= recurrent intestine) (Fig 2).
The posteriormost region of the intestine is the rectum. Upon reaching the vicinity of the esophagus the ascending intestine reverses direction and turns posteriorly to become the rectum. The rectum extends posteriorly and enters the pericardial cavity. The rectum leaves the pericardial cavity posteriorly and runs over the dorsal curve of the posterior adductor muscle to terminate at the anus in the exhalant chamber.
References
Field IA. 1922. Biology and economic value of the sea mussel, Mytilus edulis. Bull Bur. Fisheries 38:127-259.
Ruppert EE, Fox RS, Barnes RB. 2004. Invertebrate Zoology, A functional evolutionary approach, 7 th ed. Brooks Cole Thomson, Belmont CA. 963 pp.
White KM. 1937. Mytilus. Liverpool Marine Biol. Committee. 31:1-117, pls 1-30.
Supplies
Dissecting microscope
Compound microscope
Slides and coverslips
Living or preserved marine mussel
Empty shell
Screwdriver
Small dissecting pan or culture dish
Volcanic ash, chalk dust, or carmine-seawater suspension
Dye/seawater solution