Invertebrate Anatomy OnLine
Mellita quinquiesperforata ©
Sand Dollar
25may2007
Copyright 2001 by
Richard Fox
Lander University
Preface
This is one of many exercises available from Invertebrate Anatomy OnLine , an Internet laboratory manual for courses in Invertebrate Zoology. Additional exercises, a glossary, and chapters on supplies and laboratory techniques are also available at this site. Terminology and phylogeny used in these exercises correspond to usage in the Invertebrate Zoology textbook by Ruppert, Fox, and Barnes (2004). Hyphenated figure callouts refer to figures in the textbook. Callouts that are not hyphenated refer to figures embedded in the exercise. The glossary includes terms from this textbook as well as the laboratory exercises.
Systematics
Echinodermata P, Eleutherozoa, Cryptosyringida, Echinozoa, Echinoidea C, Euechinoida sC, Clypeasteroida O, Mellitidae F (Fig 9-26, 27-12, 28-62)
Echinodermata P
Echinoderms are secondarily radially symmetric deuterostomes whose ancestors were bilaterally symmetric. The adult radial symmetry is pentamerous with body parts occurring in fives or multiples thereof. Echinoderms have strong affinities with the ancestral trimeric deuterostomes especially in the tripartite organization of the coelomic cavities. Echinoderm larvae have the coelom divided into three regions, as is typical of the early coelomates, and these regions have important adult derivatives. All echinoderms are marine and benthic. About 6000 Recent species are known but the fossil record includes 13,000 extinct species.
An important echinoderm apomorphy is the water vascular system that in most groups functions in support of locomotory tube feet but is also important in gas exchange, excretion, and feeding. The body wall includes a thick connective tissue dermis in which calcareous ossicles (little bones) are almost always an important component. These ossicles make up an endoskeleton which assumes different forms in different taxa. In most echinoderms calcareous spines of various sizes and shapes arise from the dermis and extend from the body surface and are alluded to by the name echinoderm (= spiny skin). The connective tissue is mutable and its consistency is under nervous control.
Excretion in echinoderms is accomplished by simple diffusion of metabolic wastes (ammonia) across thin permeable regions of the body wall. A variety of gas exchange structures, including the tube feet, is found in various echinoderms. A hemal system is present but its role in transport is still poorly understood and the chief transport system is the circulating fluid of the various coelomic compartments. The hemal system may be through transport system that delivers nutrients from the gut to these compartments for local distribution. The nervous system consists of two central intraepidermal nerve rings from which arise radial nerves to the periphery. Echinoderms are gonochoric and fertilization is usually external.
Eleutherozoa
Eleutherozoans are mobile echinoderms in which the oral surface is oriented against the substratum. A madreporite and locomotory tube feet are present. Polian vesicles and Tiedemann’s bodies may be present on the ring canal. Movable spines are present. Eleutherozoa includes all Recent echinoderms except for its sister taxon, Crinoidea.
Cryptosyringida
Cryptosyringida includes Ophiuroidea, Echinoidea, Holothuroidea, all with closed ambulacra in which the radial nerve is internalized and protected by ossicles (Fig 28-20).
Echinozoa
Echinozoa, sister taxon to Ophiuroidea, consists of the urchins and sea cucumbers. In these echinoderms the oral surface and ambulacra have expanded aborally until they enclose almost the entire body except for a small aboral periproctal region with the anus. A bony ring of ossicles surrounds the pharynx. The hemal system is better developed than in other echinoderms. The tube feet have ossicles
Echinoidea C
Echinoidea includes about 950 extant species of sea urchins, sand dollars, sea biscuits, heart urchins, and their relatives. The dermal ossicles are thin plates fused to form a rigid, more or less spherical, endoskeletal test. Except for a thin outer epidermis, all the soft anatomy is inside the test. The larva is a bilaterally symmetrical echinopluteus. The test is covered by an abundance of movable spines. Tube feet are the major respiratory organs and the madreporite is aboral.
Urchins may be regular or irregular. Regular urchins are the sea urchins, with radial symmetry, globose nearly spherical bodies, and long spines. Most are epifaunal. Irregular urchinsare sand dollars, sea biscuits, and keyhole, heart, and cake urchins. These are usually infaunal in soft sediments and have a superficial bilateral symmetry superimposed on their radial symmetry. The body is usually flattened and the spines short.
Epifaunal species (regular urchins) possess a feeding apparatus known as Aristotle's lantern, equipped with five strong teeth, used for scraping food from hard substrates. The lantern is reduced in infaunal species (irregular urchins) because most of them are deposit feeders.
Clypeasteroida O
Clypeasteroids are flattened urchins known as sand dollars (Fig 28-42) and sea biscuits (Fig 28-41) and adapted for living and moving infaunally in soft sediments. The test is flattened to varying degrees on the oral-aboral axis and a secondary bilateral symmetry is imposed on the underlying radial symmetry. Jaws and a simplified Aristotle’s lantern are present. Respiratory tube feet are arranged in petalloids.
Strongly depressed clypeasteroids are known as "sand dollars" because some of them have a flattened disklike shape that resembles a silver dollar (Fig 28-42). Like other irregular urchins most are infaunal deposit feeders with numerous short spines that are used to burrow through soft sediments. As an adaptation to facilitate movement through sediment, irregular urchins tend to be slightly elongate and streamlined along an axis perpendicular to the oral-aboral axis. This destroys the typical echinoderm radial symmetry and replaces it with a slight bilateral symmetry (which is why they are said to be “irregular”). Notice that this is not the same as the bilaterality of holothuroids, in which the anteroposterior axis coincides with the oral-aboral axis.
The aboral ambulacral center of the clypeasteroids is at the approximate center of the aboral surface and the oral ambulacral center is at the center of the oral surface. The anus, however, does not coincide with the aboral ambulacral center (it does in regular urchins) but occupies a position on the posterior part of the oral surface low on the posterior margin of the aboral surface (Fig 28-42). The evolutionary migration of the anus away from its ancestral position at the aboral pole can be followed in fossil echinoids. In contrast, the mouth remains associated with the oral ambulacral center, and for good reason. The small tube feet of the oral ambulacra make up a set of radiating conveyor belts that transport food particles from the periphery to the central mouth.
Laboratory Specimens
Any sand dollar can be used for this exercise but it is written specifically for Mellita quinquiesperforata, the five-slotted sand dollar (or keyhole urchin). This species occurs on the American Atlantic Coast from Virginia to Brazil and is the common sand dollar of the southeastern United States. Other sand dollars are found on other coasts and some of them may be available in the laboratory for comparison. Leodia sexiesperforata (= Mellita sexiesperforata) occurs from South Carolina to Uruguay. Echinarachnius parma is the common dollar of the North American east coast from New Jersey north. It is circumpolar and also occurs in Alaska, British Columbia, Siberia and Japan. Dendraster excentricus (Fig 28- 42D) is found on the Pacific coast from Lower California to Alaska. Encope michelini, the arrowhead sand dollar (Fig 28-41B) is a tropical species common in the Caribbean and south Florida. If cleaned and dissected tests these genera are available compare them with Mellita or whatever species you are studying.
The major external differences between these taxa are in the shape of the disk, the number of lunules, the position of the lunules with respect to the disk margin, the location of the periproct, and the branching pattern of the food grooves. Echinarachnius is almost perfectly circular (like a silver dollar) and shows very little bilaterality except for the dislocation of the periproct away from the aboral pole. Dendraster is a little wider than long. Leodia has six lunules, Echinarachnius and Dendraster have none.
Most of the soft anatomy of the clypeasteroids resembles that of the regular urchins and the major differences are in the test and respiratory podia. Since most clypeasteroids are more difficult to dissect than the regular urchins, it is usually better to study the specialized features of the clypeasteroid test using cleaned dried skeletons while a regular urchin is used for the study of general urchin anatomy. On the assumption that most invertebrate zoology laboratories will study only the test, that is presented first. For those courses with access to living specimens the discussion of the test is followed by a section describing a complete, intact, living specimen.
Test
The test, or endoskeleton, like that of regular urchins, is made of fused, platelike, calcareous ossicles in the dermis of the body wall. In some individuals it is easy to see the lines of fusion between adjacent ossicles but in others they are obscure. As in other echinoids, five double rows of tube feet radiate from the center of the oral surface to the center of the aboral surface. Each double row is an ambulacrum and the areas between ambulacra are interambulacra. The arrangement of ambulacra is radially symmetrical.
Symmetry
Sand dollars are strongly depressed, or flattened, in a plane perpendicular to the oral-aboral axis (Fig 4) and are more or less disk-shaped (Fig 1, 28-42A). The oral surface is flattened or slightly concave and is the lower surface. The aboral surface is convex and is upper. The antero-posterior axis is perpendicular to the oral-aboral axis. Note the weak bilateral symmetry in addition to the more obvious radial symmetry. The anterior margin of the disk is rounded whereas the posterior is truncate, slightly emarginate (indented), or rounded (Fig 28-42A,B), depending on taxon.
Aboral Surface
Petalloids
In irregular urchins some of the tube feet of the aboral surface are respiratory whereas those of the oral surface are used for feeding and/or locomotion. The aboral surface bears five conspicuous ambulacra, called petalloids, in reference to their resemblance to the petals of a flower (Fig 1, 2, 28-42A). The areas between the petalloids are interambulacra. One petalloid points anteriorly (12:00), two of them point antero-laterally (10:00 and 2:00), and two postero-laterally (7:00 and 5:00). Under magnification you can see that each petalloid consists of two rows of paired pores which accommodate the ducts of respiratory tube feet as they pass through the test (Fig 3, 28-37C). Each tube foot has a pair of pores and the two pores of each pair are connected by a conjugation groove. In life, the groove contains the base of the respiratory tube foot served by the two pores it connects. The petalloids are simply rows of paired pores and their conjugation grooves as can be verified with magnification. Pore pairs joined by a conjugation groove are said to be conjugate.
The tube feet associated with the petalloids are respiratory and are not typical in shape or function. They are specialized gills with enhanced surface area rather than being ordinary locomotory tube feet. They do not have suckers, are not tubular, and are not used for locomotion. Their shape facilitates an efficient countercurrent gas exchange mechanism between seawater and the fluid of the water vascular system (Fig 28-37C). Respiratory tube feet are long, low, flat vesicles that lie in the conjugation groove extending from one pore to the other. They are not slender tubes extending far away from the body surface as are the more typical tube feet of regular urchins. Their long axes are parallel, perpendicular to the ambulacral axis and to the surface of the test. Their ampullae are similarly shaped but on the inside of the test (Fig 28-37C).
Figure 1. Aboral surface of the keyhole urchin, Mellita quinquesperforata. Echinoid57L.gif
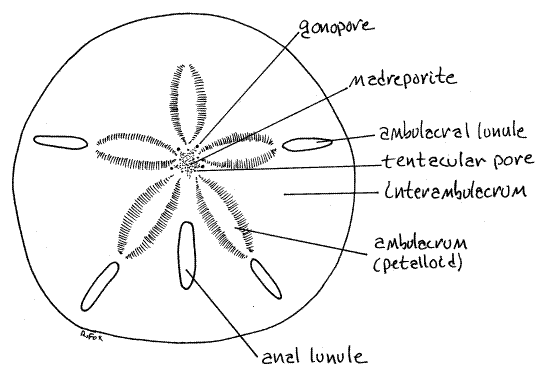
The respiratory current generated by epidermal cilia flows over the surface of the tube foot from the midline of the petalloid toward its side (Fig 28-37C). The ciliated peritoneum of the water vascular system inside the tube foot moves a current in the opposite direction, from the side toward the midline. Oxygen, following its gradient, diffuses into the tube foot from the seawater along the entire area of contact between tube foot and seawater. Oxygenated water in the tube foot then flows through the test via a median podial duct into the ampulla. Once inside the ampulla, oxygen is transferred to the fluid of the perivisceral coelom by another countercurrent mechanism, this one between perivisceral coelom and water vascular system. The fluid in the ampulla, now depleted of oxygen flows back through the test through the second of the podial pores, this one lateral to the petalloid. Of the two podial pores flow is from tube foot to ampulla in the medial pore and ampulla to tube foot in the lateral pore.
Lunules
The body is perforated by five (six in some species, none in others) slots, or lunules that pass entirely through it. In Mellita quinquiesperforata an ambulacral lunule is situated near the periphery of the disk in each of the five ambulacra except the anterior one (Fig 28-42A,B). A fifth lunule, the anal lunule, is closer to the center and is located in the posterior interambulacrum. In juvenile dollars the lunules begin as notches in the disk margin which deepen and then close as the animal grows (Fig 28-41B). Some young dollars may be available to demonstrate this condition.
Figure 2. One of the five petalloids of the aboral surface of Mellita. Echinoid58L.gif
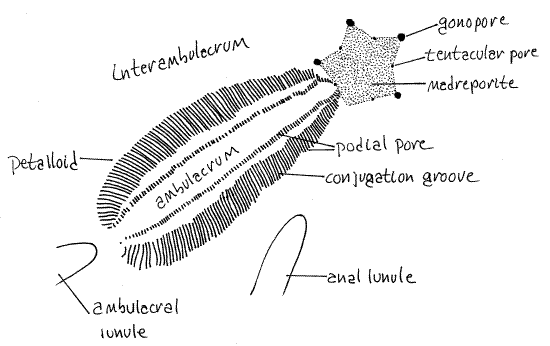
Lunules are not present in some mellitids, such as Dendraster and Echinarachnius or in clypeasterids. (Leodia and Encope (Fig 28-41B) have five ambulacral and one interambulacral lunule for a total of six. The ambulacral lunules of Encope are confluent with the margin of the dollar and are deep notches in the margin rather than holes in the disk. The anal, or interambulacral, lunule of Encope is not confluent with the margin and is a complete hole rather than a notch. Encope resembles an arrowhead. All the lunules of Leodia are holes, not notches.)
Aboral Ambulacral Center
Look at the center of the aboral ambulacral center with the high power of your dissecting microscope (Fig 1, 2, 28-42A). It is slightly anterior to the center of the aboral surface. (The aboral ambulacral center of Dendraster is displaced a little posteriorly from center. As a consequence, the three anterior petalloids are longer than the two posterior.)
The madreporite is a star-shaped area at the ambulacral center. Its surface is perforated with tiny madreporic pores. In life these pores are lined with a ciliated epithelium and connect with the axial canal and stone canal of the water vascular system.
Nine pores penetrate the test around the periphery of the madreporite (Fig 2). A gonopore is situated at the apex of four of the five points of the madreporite. The gonopores are interambulacral. The posterior interambulacrum lacks a gonopore. (Encope has five gonopores, one in each interambulacrum). The remaining five openings around the madreporite are ambulacral and are tentacular pores for the exit of the terminal portion of the radial water canal, which is called a tentacle.
Oral Surface
Examine the oral surface with your unaided eye (Fig 3). The lunules are, of course, evident on this side also since they pass completely through the body. In contrast the mouth and anus penetrate only one wall, as do podial pores, gonopores, and tentacular pores.
Figure 3. The oral surface of Mellita. Echinoid59L.gif
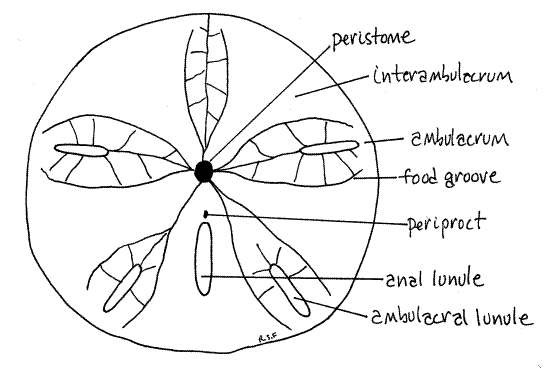
Peristome
The center of the oral surface is the relatively small peristome. The peristomial aperture, which in life would have the mouth at its center, is a circular opening located a little anterior to the center of the oral surface. It is covered by the peristomial membrane in living dollars. The peristome coincides with the oral ambulacral center from which radiate five ambulacra, known as phyllodes.
The five narrow, incised food grooves, or ambulacral furrows, arise singly from the margin of the peristome but each quickly branches to form two food grooves that run beside the ambulacral axes, on either side of the lunules. (The food grooves of Echinarachnius are long and straight and do not branch until they near the periphery of the disk.)
Periproct
The periproct, which in life surrounds the anus, is a small opening in the posterior interambulacrum between the peristome and the anal lunule (Fig 28-42B). It is near the mouth on the oral surface. (The periproct of Echinarachnius is located at the center of the posterior margin of the disk.)
Tubercles
Study the oral and aboral surfaces with high power of the dissecting microscope. Most of the surface is covered with tubercles located in shallow pits. In life, each tubercle articulates with a movable spine whose motion is controlled by a ring of muscles running from the test to the base of the spine (Fig 28-32A). Compare the size of the tubercles (and by inference the size of their spines) on the aboral surface, oral surface, and margins of the lunules. Where are the spines the largest? Look at the demonstration of a test that is still covered with spines.
Podial Pores
With high power of the dissecting microscope, search the oral surface for the unpaired pores of suckered tube feet. These are conventional, albeit very small, tube feet with suckered tips. Each is served by a single podial duct from its ampulla. The tube feet emerge from the test via tiny pores. The pores are small but can be seen if you adjust the angle of the incident light. The suckered podia are most abundant on the oral surface in the food grooves. They are organized in leaf-shaped ambulacra known as phyllodes (Fig 28-42B). (The pores are easier to see in Mellita than in Clypeaster.)
The pigmentation pattern sometimes evident on the oral surface of dried tests is due to the uneven distribution of large and small pitted tubercules and unpaired pores. The darker areas surrounding the ambulacra and food grooves are areas of small tubercles and abundant podial pores. The lighter interambulacral areas bear much larger tubercles and few or no tube feet. The same pattern can be seen in dollars that still have their spines. Larger spines are in the interambulacra and smaller spines in the ambulacral areas of the oral surface.
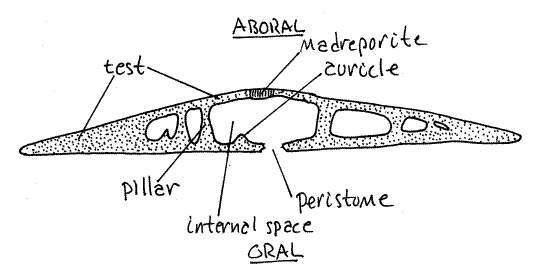
Bisected Test
Examine the demonstration of a sand dollar test bisected along the anterior-posterior axis (Fig 4). The test is easily sectioned with a hacksaw but this will probably be done for you prior to class. Note the internal structural support. It is best developed around the periphery where the test is almost solid. The central region is largely open and it is here that the viscera are located (in living sand dollars). Even near the central region, however, skeletal pillars extend between the upper and lower walls of the test.
Aristotle's Lantern
In regular urchins Aristotle’s lantern is a complex arrangement of five jaws, their teeth, and muscles in the center of the body. It is an internal structure requiring dissection for its study. If you plan to dissect a sand dollar, postpone study of the lantern until you have opened your specimen. If not use a dried dissected specimen provided by the staff. In either case, use the following description.
The lantern of clypeasteroids, which are deposit feeders, is much simpler than that of regular urchins, which are browsers, and essentially consists of five jaws holding five teeth (Fig 1). The rotulae, and epiphyses are absent or vestigial. Each jaw consists of a pyramid and a compass. Five triangular ossicles revealed by breaking open a sand dollar test are sometimes called "doves" by the beachcombing public because they resemble birds with spread wings. The doves are the pyramids of the lantern. The teeth may be lost in dried preparations.
Mellita is not a good choice for study of lanterns. A regular urchin should be used for a careful study of a typical urchin lantern. For study of the lantern of irregular urchins, Clypeaster (Invertebrate Anatomy OnLine ) , because of its large size, is much better than Mellita.
Figure 4. A transverse section of Mellita. Echinoid60L.gif
Living Specimen
If living dollars are available place one in a dish or pan of seawater. If a preserved animal is used it should be placed in tap water. Examine your specimen with the dissecting microscope.
External Anatomy
The oral and aboral surfaces are covered with a dense felt of short spines (Fig 28-42C). These are much more numerous and much smaller than those of regular urchins. Do the spines appear to be uniform in size or is there more than one size? Compare the arrangement of the club-shaped spines of the petalloids with those elsewhere (Fig 28-42F). In the petalloids they are arranged in straight rows. Look for signs of the thin epidermis that covers each spine. Look between the spines for pedicellariae. How many jaws do the pedicellariae have? Are they stalked?
>1a. Under magnification touch the aboral surface with a needle and watch the response of the nearby spines. <
On the aboral surface find the five petalloids, or ambulacra. Each consists of two rows of respiratory tube feet. These podia are low, lamellae running perpendicular to the radial ambulacral axis of the ambulacrum (Fig 2, 28-37). Each petalloid has two rows of tube feet (Fig 28-42A). They extend between the rows made by the spines. Watch for the movement of coelomocytes, red with hemoglobin, in the respiratory tube feet. Does the current move toward or away from the radial axis of the petalloid?
Turn the dollar over and look at the center of the oral surface. The region around the mouth is the peristome (Fig 3). The peristomial aperture, which you cannot see because it is covered by the peristomial membrane, is at its center. The mouth, which you can see, is in the center of the peristomial membrane. Note the longer spines that guard the opening. Examine the five food grooves radiating from the mouth. Abundant suckered tube feet, but no spines or pedicellaria, are present in the grooves (Fig 28-43). Other suckered tube feet are located amongst the spines of other parts of the surface.
Find the periproct on the oral surface between the mouth and the anal lunule on an interambulacral axis. The anus is atop a conical papilla, which is barely shorter than the long spines around it. (The periproct of Echinarachnius and Dendraster are on the posterior margin (Fig 28-42D)).
>1b. Compare the spines of the oral surface with those of the aboral surface. Are they different? If so, do their differences correlate with different functions? <
Internal Anatomy
The hard test and small, crowded test cavity of sand dollars make for a difficult, but by no means impossible, dissection. In introductory laboratories it is preferable to study the internal anatomy of echinoids using a regular urchin. Advanced courses or courses taught on the coast may, however, find it worthwhile to dissect a sand dollar. Living specimens should be in isotonic magnesium chloride for the dissection. Preserved specimens should be in a pan or culture dish of tapwater.
" To open the test, remove the dollar from the water and place it on your workbench against a firm flat surface. Use the following instructions to make a small opening in the test of the aboral surface somewhere off center so you miss the madreporite. Do not damage the soft tissues within and be careful throughout to avoid damage to the stone canal and axial hemal vessel (=axial gland) in the center of the animal, below and attached to the madreporite.
The small initial opening can be made by applying the tip of a scalpel to the surface and tapping the handle with a blunt probe or some other makeshift mallet. The scalpel is being used as a chisel and will be ruined by the process so use an old blade. Cut a square opening in the test by making four cuts with the "chisel". The object is to make an opening to admit one blade of a pair of bone snips or needle-nose pliers. The first chisel cut will take the longest; be patient and keep tapping.
When the point of the scalpel penetrates the test, move it and start another cut at a right angle to the first. Continue this until you have made a little square window in the test. Remove the plug of test and insert one blade of a pair of small bone snips or fine needle nose pliers into the opening. Use them to grasp and break away the margins of the window.
Enlarge the window gradually in this fashion but always be careful of the soft structures inside. Try to keep the stone canal intact and attached to the madreporite but don't be dismayed if you are unable to do so. Continue expanding the opening towards the periphery of the disk until you reach the thin marginal part of the disk where there are no soft parts (Fig 4). Replace the dollar in the dissecting pan of magnesium chloride and wash or pick away the adhering chips of skeleton.
Figure 5. Aboral dissection of the keyhole urchin, Mellita quinquiesperforata. The test has been removed to reveal the soft anatomy and Aristotle's lantern. The direction of flow in the gut is indicated with arrows. Echinoid13La.gif
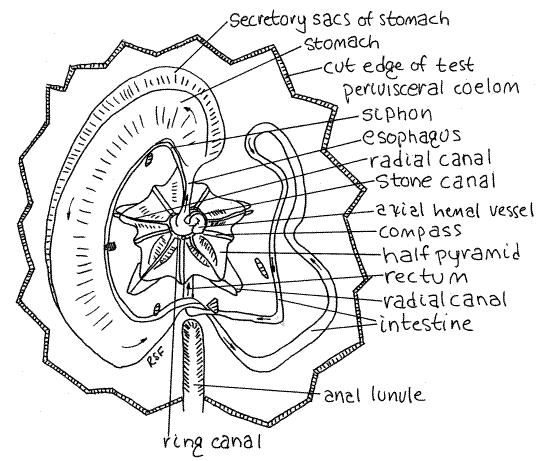
Preview
If you have been careful in your dissection so far, what you see as you look into the opening is the white, pentagonal Aristotle's lantern (Fig 5) in the center and four large, purplishgonads surrounding it. Not much else will be visible now except for the esophagus emerging from the center of the lantern and the dark axial hemal vessel (= axial gland) beside it. These will be hidden from view by the madreporite if you were successful in retaining it.
Carefully turn the madreporite and associated test over and locate the axial hemal vessel and esophagus. Four gonoducts from the four gonads extend to their respective gonopores on the genital plates around the madreporite. These originate as highly branched tubes in the gonads.
Aristotle's Lantern
The lantern consists of five blocky pyramids which hold the five slender teeth (Fig 5). The teeth are on the interambulacral axes but are difficult to see from your viewpoint. The radiating points of the pyramids are on the ambulacra. The compasses (or radii) are well developed in Mellita. They are slender rods radiating outward from the center on the aboral surface of the lantern. The remaining parts of the typical urchin lantern are vestigial or absent. The lantern is enclosed in its own peripharyngeal coelom surrounded by the peripharyngeal peritoneum.
Water Vascular System
Look at the center of the lantern with 30X. The ring canal of the water vascular system can be seen as a slightly translucent ring around the esophagus at the top (aboral) end of the lantern (Fig 5). (In Echinarachnius the ring canal is a dark doughnut encircling the esophagus deep in the core of the lantern.) The stone canal arises from the ring and is surrounded by the dark green axial hemal vessel , which is part of the hemal system. The stone canal runs to the madreporite. Five radial canals emerge from the ring canal and are easily seen on top of the pyramids, on the ambulacral axes. They run peripherally out to the points of the pyramids where they turn orally and disappear from view. You will see them again later. Five Tiedemann's bodies can be seen as oblong patches of cells on the outer wall of the water ring. They are interambulacral.
Reproductive System
The four, not five, gonads are obvious but may be difficult to distinguish from each other. Before you removed the test, each of them connected via a gonoduct to a gonopore at the aboral pole. Look for the branching gonoduct leading from the interior of each gonad toward the aboral pole.
Digestive System
Locate the esophagus where it emerges from the center of the lantern and be careful that you do not damage it (Fig 5). Carefully remove the gonads without harming the wide delicate intestine oral to them. Do this under magnification, carefully breaking the connections between the gonads and the gut. This may be tedious as the gonad sometimes adheres tenaciously to the intestine.
The mouth on the oral surface opens into a short pharynx contained within the core of the lantern. It is not visible without opening the lantern. As the gut exits the lantern it becomes the short esophagus which extends to the side from the center of the lantern. It is on an ambulacral axis (Fig 5). It joins the blind end of a very wide, flat stomach. The outer border of the stomach is lobed with secretory sacs.
The stomach initiates a counterclockwise loop that takes the gut halfway around the periphery of the test back to the anal lunule. A conspicuous, but small-diameter, tubular siphon exits the esophagus at its junction with the stomach and parallels the inner curve of the stomach before eventually rejoining the stomach at its junction with the intestine (Fig 5).
The stomach ends and the intestine begins at the point where the siphon rejoins the gut beside the anal lunule (Fig 5). The intestine, which is much narrower than the stomach, continues counterclockwise around the periphery of the disk after curving centrally to avoid the anal lunule. (The intestine of Echinarachnius does not make such a curve as there is no lunule to avoid.) The intestine continues counterclockwise until it reaches about 2:00 near the junction of the stomach and esophagus at the anterior end of the urchin. The intestine then reverses direction and returns clockwise to the anal interambulacral axis where it passes aboral and then oral to itself and runs anteriorly to the anus in the periproct just posterior to the mouth. (The intestine of Echinarachnius runs to an anus on the posterior border of the disk.)
Remove the gut from the remains of the test and find the five radial canals extending across the floor of the test on the ambulacral axes.
Nervous and Hemal Systems
Dissection of the nervous and hemal systems is not practical in sand dollars.
References
Coe WR . 1912. Echinoderms of Connecticut. State Geol. Nat. Hist. Survey Bull. 19:1-152.
Hyman LH. 1955. The Invertebrates, vol. 4 Echinodermata. McGraw-Hill, New York. 763 pp.
Ruppert EE, Fox RS, Barnes RB. 2004. Invertebrate Zoology, A functional evolutionary approach, 7 th ed. Brooks Cole Thomson, Belmont CA. 963 pp.
Smiley S. 1994. Holothuroidea, pp. 401-471 in Harrison FW, Chia FS (eds.). Microscopic Anatomy of Invertebrates vol. 14 Echinodermata. Wiley-Liss, New York. 510p.
Supplies
Test
Dissecting microscope
Cleaned sand dollar test
Test with spines intact
Bisected test
Dissected dried dollar with lantern
Living Specimen
Living sand dollar
Dissecting pan
Seawater
Isotonic magnesium chloride
Bone snips or needle nose pliers
Dissecting microscope with microdissecting tools