Invertebrate Anatomy OnLine
Ilyanassa obsoleta ©
Mud Snail
3jul2006
Copyright 2003 by
Richard Fox
Lander University
Preface
This is one of many exercises available from Invertebrate Anatomy OnLine , an Internet laboratory manual for courses in Invertebrate Zoology. Additional exercises can be accessed by clicking on the links to the left. A glossary and chapters on supplies and laboratory techniques are also available. Terminology and phylogeny used in these exercises correspond to usage in the Invertebrate Zoology textbook by Ruppert, Fox, and Barnes (2004). Hyphenated figure callouts refer to figures in the textbook. Callouts that are not hyphenated refer to figures embedded in the exercise. The glossary includes terms from this textbook as well as the laboratory exercises.
Systematics
Mollusca P, Eumollusca, Conchifera, Ganglionura, Rhacopoda, Gastropoda C, Prosobranchia sC, Caenogastropoda O, Neogastropoda sO, Muricoidea SF, Nassariidae F (Fig 12-125)
Mollusca P
Mollusca, the second largest metazoan taxon, consists of Aplacophora, Polyplacophora, Monoplacophora, Gastropoda, Cephalopoda, Bivalvia, and Scaphopoda. The typical mollusc has a calcareous shell, muscular foot, head with mouth and sense organs, and a visceral mass containing most of the gut, the heart, gonads, and kidney. Dorsally the body wall is the mantle and a fold of this body wall forms and encloses that all important molluscan chamber, the mantle cavity. The mantle cavity is filled with water or air and in it are located the gill(s), anus, nephridiopore(s) and gonopore(s). The coelom is reduced to small spaces including the pericardial cavity containing the heart and the gonocoel containing the gonad.
The well-developed hemal system consists of the heart and vessels leading to a spacious hemocoel in which most of the viscera are located. The kidneys are large metanephridia. The central nervous system is cephalized and tetraneurous. There is a tendency to concentrate ganglia in the circumenteric nerve ring from which arise four major longitudinal nerve cords.
Molluscs may be either gonochoric or hermaphroditic. Spiral cleavage produces a veliger larva in many taxa unless it is suppressed in favor of direct development or another larva. Molluscs arose in the sea and most remain there but molluscs have also colonized freshwater and terrestrial habitats.
Eumollusca
Eumollusca, the sister taxon of Aplacophora, includes all molluscs other than aplacophorans. The eumolluscan gut has digestive ceca which are lacking in aplacophorans, the gut is coiled, and a complex radular musculature is present.
Conchifera
Conchifera, the sister taxon of Polyplacophora, includes all Recent molluscs other than aplacophorans and chitons. The conchiferan shell consists of an outer proteinaceous periostracum underlain by calcareous layers and is a single piece (although in some it may appear to be divided into two valves). The mantle margins are divided into three folds.
Ganglioneura
Most Recent molluscs are ganglioneurans, only the small taxa Aplacophora, Polyplacophora, and Monoplacophora are excluded. Neuron cell bodies are localized in ganglia.
Rhacopoda
The mantle cavity is posterior in the ancestor although it may be secondarily moved to an anterior position by torsion. This taxon includes gastropods and cephalopods.
Gastropoda C
Gastropoda is the largest molluscan taxon and is the sister group of Cephalopoda. Gastropods are united by descent from a torted ancestor although many exhibit various degrees of detorsion. Many are coiled and asymmetrical but the ancestor was probably symmetrical. Gastropods are relatively unspecialized molluscs known collectively as snails. The univalve shell, present in the ancestral gastropod and in the majority of Recent species, is reduced or lost in many representatives. The flat creeping foot was inherited from their eumolluscan ancestors but gastropods have developed a distinct head with an abundance of sophisticated sense organs. The originally posterior mantle cavity has become anterior as a consequence of torsion, although detorsion has reversed this condition in many. Gastropods were originally gonochoric and most remain so but many derived taxa are hermaphroditic. Most are marine but many taxa have invaded freshwater and the only terrestrial molluscs are gastropods. Most have a single gill, atrium, and nephridium but the most primitive representatives have two of each. Only one gonad, the right, is present. The ancestor probably had an operculum. The nervous system is streptoneurous (twisted by torsion).
Prosobranchia sC
Prosobranchia was once one of three great gastropod subclasses but it is no longer considered to be a monophyletic taxon, although the concept continues to be used as a pedagogical convenience. Prosobranchs are the gastropods most like the ancestral snails. They are torted and most have a shell and are coiled and asymmetrical. The mantle cavity is anterior. Most are gonochoric and most have an operculum. Most have only one gill in the mantle cavity but some primitive taxa have two. The right atrium is lost in most. Prosobranchs are specialized for life in marine benthic habitats although representatives are also found in freshwater and on land.
Caenogastropoda O
Caenogastropoda includes the two large and successful groups, mesogastropods and Neogastropoda. One gill, one nephridium, and one atrium are present. The gill is monopectinate, with filaments on only one side of the central axis. This new gill is less prone to fouling with sediment and silt and is probably largely responsible for the success of these snails as it allowed invasion of soft-bottom habitats.
Neogastropoda sO
Neogastropods are the modern marine snails. They have a well-developed, gill-like, bipectinate osphradium in contrast with the much simpler osphradium of mesogastropods. The rachiglossate radula has three teeth in each transverse row whereas that of mesogastropods has seven. A gland and valve of Leiblein are present in the gut. Most are carnivores and all are marine. Neogastropoda includes many well-known gastropods such as the tulip snails, whelks, conchs, oyster drills, mud snails, olive snails, and cones.
Laboratory Specimens
For many reasons small snails, such as Ilyanassa, are preferable to large species, such as Busycon, for the study of gastropod anatomy. Small species are much easier to manipulate on the stage of a dissecting microscope, are much easier to relax, are easier to obtain in large numbers, and are less expensive. Activity and behavior of unrelaxed, living gastropods is best studied using small species less than 2 cm in length.
The mud snail, Ilyanassa obsoleta (formerly Nassarius obsoletus) is a common intertidal mollusc found on both coasts of North America from Labrador to northeast Florida and Vancouver to central California. It inhabits silty or muddy sand, low energy beaches where it may occur in large aggregations (Fig 12-48A). It is ideal for the laboratory study of anatomy and behavior of prosobranch gastropods
Ilyanassa is often found in dense herds of uniformly aged individuals (Fig 12-48A). The snails respond positively to the scent of conspecifics by following their mucus trail and this results in aggregation. These snails can be very common on muddy sand low-energy beaches in salt marshes and along tidal creeks.
Ilyanassa is the intermediate host of a blood fluke (Austrobilharzia variglandis, Platyhelminthes: Trematoda) whose definitive host is a seagull. The fluke cercaria (Fig 10-35) are released from the snails into shallow water where they may inappropriately penetrate the skin of wading or swimming humans. They are unsuccessful in this attempt and cannot enter the blood and do not complete the life cycle in humans. Before dying, however, they cause a dermatitis known as swimmer's itch. (The cercaria of other flukes can also be responsible for swimmer's itch in both fresh and salt water.)
This exercise is written for living specimens but can also be used with preserved material.
External Anatomy
Shell
Obtain a living (or preserved) mud snail and scrub its surface clean with a toothbrush. Observe its external anatomy with a dissecting microscope. The animal is enclosed in a coiled, asymmetrical, univalve shell (Fig 1, 12-27A,B). The shell has a single opening, or aperture, from which the animal extends its head and foot. The aperture is elongated anteriorly to form asiphonal canal, or siphonal notch, through which a siphon passes when it is deployed. At present, if you are handling the snail, the head and foot are probably retracted and the aperture is blocked by the operculum (Fig 1, 12-27C-F), which is a thin disc of the protein conchiolin.
The shell is composed of a series of coils, or whorls, around a central axis, the columella, which cannot be seen at present. The first whorl includes the aperture and contains head, foot, and most of the visceral mass. This is the body whorl (Fig 1, 12-27A,B) and it is much larger than any other whorl. Above the body whorl are several smaller whorls. Each whorl is a complete revolution of the shell around the axis. The combined smaller whorls make up the spire (Fig 1, 12-27B). The spire sits atop the body whorl and tapers to a blunt point.
Figure 1. The shell of Ilyanassa obsoleta. Gastrop152L.gif
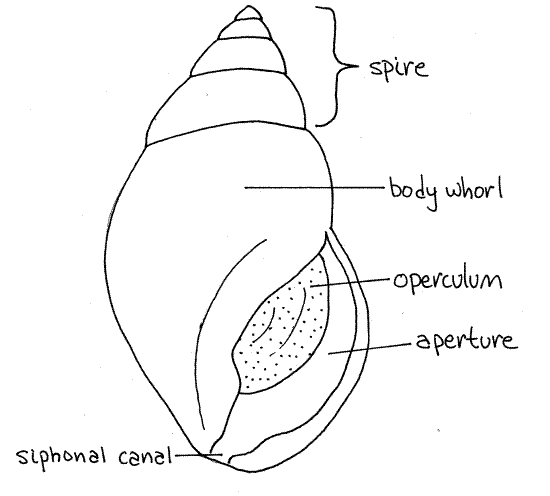
The shell is composed chiefly of calcium carbonate but a significant organic component is present as well. The outermost shell layer is a thin layer of the protein conchiolin. This outer organic layer is the periostracum. It is the dark outer covering you see as you observe the shell. The periostracum may be eroded in places, especially at the tip of the spire, to reveal the underlying chalky white prismatic layer. The smooth, lustrous lamellar layer will not be visible until you crack the shell and can see its inner surface. The prismatic and lamellar layers are composed of calcium carbonate with generous amounts of collagen.
Hold the shell so the aperture is facing you and the spire points up as in Figure 1. Determine if the aperture is on the right or the left of the central axis. If it is on the right, the snail isdextral, or right handed. If on the left, it is sinistral, or left-handed. Is your mud snail sinistral or dextral? ________ Most gastropods are dextral.
Head
If your snail is living, place it in a culture dish of seawater on the stage of the dissecting microscope. Wait for the snail to extend its foot and head, and observe the externally visible features of its anatomy as it crawls across the dish. If is preserved, study the portions of the soft anatomy extending from the aperture.
The snail body consists of head, foot, and visceral mass enclosed in the shell. The foot and head emerge from the shell when the snail is active but the visceral mass remains inside.
Find the head at the anteriormost end of the snail. This is the leading end when the snail is crawling (Fig 12-14A). It bears a pair of conspicuous, sensory, cephalic tentacles (Fig 2). A dark eye is situated lateral to the junction of each tentacle with the head.
Look for a small pore on the anterior surface between the bases of the tentacles. This is the proboscis pore through which the proboscis, with the mouth at its tip, is extended during feeding (Fig 2, 12-14). The proboscis pore is the external opening of a deep invagination, The pore is not the mouth. The proboscis is a long extensible tube containing the anterior end of the gut including the mouth, buccal cavity, radula, and anterior esophagus (Fig 12-44). It can be extended or withdrawn by muscles in the head.
Note the elephant trunk-like siphon emerging from the head dorsolaterally on the left side (Fig 2, 12-14A). It passes through the siphonal canal in the aperture of the shell. The siphon is a rolled extension of the mantle skirt and is open along one edge (Fig 12-25B). It is not a closed tube. The siphon is the inhalant canal that brings water into the mantle cavity with its sensory receptors, and gill.
>1a. While watching with magnification, offer your specimen the tip of your finger or a small piece of shrimp and observe the proboscis. Watch the proboscis as it emerges from the proboscis pore and is extended toward the food. The mouth is at the tip of the extended proboscis. Watch for the toothed rasp-like radula which may be extended from the mouth to scrape up bits of food. Watch the rocking motion of the radula. You could also use a 10X magnifier to observe snails feeding on the glass walls of aquaria, and note the activity of the radula through the glass. <
>1b. Make a shrimp sandwich by placing a small piece of shrimp, or similar protein, between two clean glass microscope slides. The shrimp should be about 3 mm from a long edge of the slides. Place a rubber band around one end of the sandwich to hold the two slides together. Place the sandwich in a 4" finger bowl of seawater with a snail. Be sure the piece of shrimp is immersed water. Watch as the snail uses its chemoreceptive equipment, especially the siphon, to locate the sandwich. The snail will probably insert its proboscis between the two slides to gain access to the bit of meat in the center. Watch the process with your dissecting microscope and pay particular attention to the radula which will be repeatedly everted and withdrawn from the mouth at the tip of the proboscis. <
The head is joined to the foot and visceral mass by a short neck. Arching over the neck is the mantle skirt (= mantle collar, mantle flap) which encloses the lip of the shell aperture (Fig 2, Fig 12-14). The skirt is the free anterior edge of the mantle. Between it and the neck is the opening into the mantle cavity. The mantle and mantle cavity will be studied later.
Figure 2. Dorsal view of Ilyanassa drawn as if it had a transparent shell. The proboscis is extended. The proboscis and siphon are extended. Redrawn from Brown (1969). Gastrop147La.gif
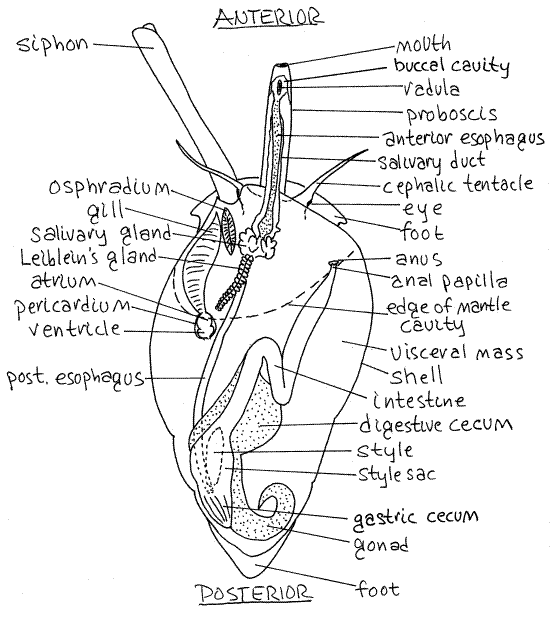
Foot
Find the broad, flat, muscular foot upon which the animal crawls (Fig 2, 12-14). The foot is ventral. The head is anterior to the foot and the visceral mass and shell are dorsal to it.
Note the thin, flat, oval, proteinaceous operculum on the dorsal surface of the foot immediately posterior to the shell. The operculum functions as a door to close the aperture (Fig 12-27C-F).
>1c. Push the snail with a blunt probe or your finger and watch it retract its head, then foot, and then close the aperture with the operculum (Fig 12-27C-F). Place the snail in the dish with the aperture up and watch patiently as the head and foot emerge from the aperture. Watch the animal attempt to right itself with its foot. <
Extraction
" Remove the shell from your specimen. Snails are attached to their shell by a strong columellar muscle that extends from the foot to an insertion high on the columella inside the shell (Fig 3,4). This is the muscle used by the animal to withdraw the head and foot into the shell. It is homologous to an ancestral pedal retractor muscle but only the right columellar muscle is present in adult snails. The insertion is deep within the shell and is not visible or accessible in an intact snail. To remove the animal from the shell it is necessary that you detach the columellar muscle from the columella and then unwind the snail out of its shell but first you must gain access to the muscle. Be very careful as you do this because the visceral mass is very fragile and must not be damaged. Do not attempt to pull the snail from the shell until the columellar muscle is detached.
Use a vise or 4-inch C-clamp to apply controlled pressure to the body whorl until it cracks. Remove any pieces of shell that move freely. Reapply the vise and continue cracking the shell and removing pieces until the columella and columellar muscle are revealed in the center of the shell (Figs 3, 4). Apply pressure only to solid, intact regions of the shell, never to loose pieces. Instead, remove the loose pieces with forceps. The white columellar muscle extends from its insertion on the foot to its origin on the columella. It is the only physical attachment between the animal and its shell. With your fine forceps, separate the muscle from the columella. The attachment should be scraped, not cut, from the columella with the forceps.
When you are sure the muscle is completely free of the columella, carefully attempt to unwind the animal out of the remains of the spire. If the columellar muscle has been completely detached, the animal can be extracted intact by this method. It may sometimes be necessary to return to the vise for more cracking but be careful that you do not apply pressure to any soft parts. Your good humor for the rest of the period depends on your success in extracting the specimen from its shell intact. It is difficult to recognize the anatomic features of a damaged visceral mass. Place your extracted specimen in a small dissecting pan or small culture dish of isotonic magnesium chloride.
Preview
Examine the extracted animal carefully with your dissecting microscope and remove any remaining pieces of shell. Orient yourself with respect to this asymmetrical, coiled, and torted animal. Be sure you know dorsal, ventral, anterior, posterior, right, and left. The head is anterior, the foot ventral, and the visceral mass dorsal. The head and foot are bilaterally symmetrical and are not coiled. The visceral mass is coiled and asymmetrical.
Relocate and review the structures found earlier. Look again for the proboscis, if it is extended, or the proboscis pore if the proboscis is retracted. Distinguish between the dark ventral foot and the lighter dorsal visceral mass. Note that the visceral mass is coiled and consists of several whorls that fit into the whorls of the shell (Fig 12-14B). The largest whorl of the visceral mass occupies the body whorl.
>1e. Look through the thin body wall into the visceral mass for short, worm-like, rod-shaped sporocysts or rediae which may be present (Fig 10-35B,C). These are larval stages of a trematode flatworm that parasitizes these snails. If you find some, make a small break in the body wall and remove a few larvae to a microscope slide. Make a wet mount and examine it with the compound microscope. Note the next generation of larvae (asexually produced) inside the redia. These are cercariae (Fig 10-35D,E). Try to rupture one of the rediae and release the cercariae inside. Each cercaria is capable of infecting the next host. <
Mantle and Mantle Cavity
In molluscs the dorsal body wall is referred to as the mantle and some part of it is folded, or invaginated, to form a pocket, or recess, which is the mantle cavity. In neogastropods torsion has moved the originally posterior mantle cavity to an anterior position above the head. In your specimen, the conspicuous fold of tissue dorsal to the neck is the part of the mantle that has been folded to form the roof of the mantle cavity. The free, unattached border of the roof is the mantle skirt (Fig 3,4). The roof is a double layer of mantle. The floor of the mantle cavity is another part of the dorsal body wall and is also the roof of the hemocoel which lies ventral to it. The siphon is a long rolled extension of the anterior, left, dorsal border of the mantle skirt.
Look for a hatchet-shaped penis emerging from beneath the anterior mantle margin on the right side (Fig4). If your specimen has a penis, it is probably a male (but females sometimes have them too).
Lift the mantle skirt with a blunt probe to reveal the mantle cavity. Look into the cavity to find the featherlike gill on the left side.
" Use your finest scissors to open the mantle cavity with a mid-dorsal incision extending posteriorly from the mantle margin. The incision should be along the right margin of the gill. Deflect the cut edges of the mantle and look on the right for the large anal papilla with the anus at its distal tip (Fig 2, 12-14B). If you have a male, the penis will be on the right side and if you have a female look for the small genital papilla medial and posterior to the anal papilla.
The single gill is located on the left floor of the mantle cavity (Fig 2, 12-14B). It is connected to the heart by an efferent vessel. The heart lies in the pericardial cavity located just posterior to the gill (Fig 2). The heart consists of a single atrium and ventricle but it is difficult to find in these small animals. Look for the oval osphradium between the anterior end of the gill and the siphon (Fig 2). The osphradium resembles a miniature gill but is bipectinate whereas the neogastropod gill is monopectinate. It is a sense organ that detects silt in the inhalant water flow from the siphon. Water flows over the osphradium before flowing over the gill.
>1d. If your snail is living, place a drop of dye (in sea water) in front of it and watch the pigment enter and leave the mantle cavity. Note the pattern of flow of the dye. In neogastropods water enters through the siphon on the left, flows across the osphradium, gill, nephridiopores, and anus to exit on the right. <
Reproductive System
Like most prosobranch gastropods, Ilyanassa is gonochoric. Most of the reproductive system is visible without dissection and is best studied before opening the body cavity. In both sexes the gonad lies at the apex of the visceral mass and connects with a gonopore via a long gonoduct on the right side of the body (Figs 3, 4, 12-14B). The gonoduct is regionally specialized in both sexes and is similar to that of other neogastropods (Fig 12-56).
Study of the reproductive system requires adult specimens in reproductive condition. Reproductive males have a large, non-retractable, hatchet-shaped penis in the right side of the mantle cavity Fig 4). Examine this area to determine the sex of your specimen. Females usually do not have a penis but do possess a large white swelling, the egg capsule gland, on the right side of the visceral mass and mantle cavity. Study the reproductive systems of both sexes.
Female
The large cream-colored ovary at the apex of the visceral mass is surrounded by the greenish-brown digestive ceca (Fig 2,3). Eggs produced by the ovary pass through the posterioroviduct located close to the surface on the right side of the visceral mass. This portion of the oviduct is a yellow, straight, narrow, transparent tube extending to the region of the columellar muscle. Individual eggs can sometimes be seen in the oviduct. It is difficult to see if it does not contain eggs.
Anteriorly, the oviduct is regionally specialized to form several structures, some with unknown function. The posterior oviduct opens into the large white albumen gland which adds a layer of albumen to the zygote before it reaches the egg capsule gland.
The rusty brown seminal receptacle (= sperm ingesting gland) is located between the albumen gland and the egg capsule gland. Its function is uncertain but it may store sperm prior to fertilization or it may destroy superfluous sperm.
From the albumen gland eggs pass next to the egg capsule gland (Fig 3). This is the largest and most conspicuous region of the oviduct. It is oblong and white. It is also known as the egg case gland and it secretes liquid, malleable, proteinaceous capsules around the eggs after they have been fertilized.
The egg capsule gland opens via a short duct to the obscure female gonopore located at the tip of the short genital papilla located proximal to the base of the much longer anal papillaon the roof of the right mantle cavity (Fig 2). The gonopore is a slit that is closed except when an egg capsule is being released. If there is a capsule in the oviduct it can be seen through the transparent body wall and can be squeezed out through the gonopore with gentle pressure from your forceps. This is the most reliable way to find the gonopore.
Eggs, surrounded by the protein egg case exit the gonopore and are transferred to the pedal gland where the still malleable protein is molded into a species-specific shape and attached to an appropriate substratum. The pedal gland is a recess, or pocket, in the center of the anterior quarter of the sole of the foot.
At the end of the reproductive season the ovary shrinks and becomes darker. The posterior oviduct atrophies and becomes difficult to see. The albumen gland and egg capsule gland loose their white color and become gray-tan.
Male
The bright red-orange testis is located at the apex of the visceral mass and is surrounded by the large, greenish-brown digestive ceca (Fig 4, 12-56B). The male gonoduct exits the testis on its anterior right margin and extends anteriorly past the columellar muscle to the mantle cavity and then to the penis.
The gonoduct is regionally specialized and consists of regions derived from the coelomoduct of the gonocoel, the right renal duct, and the mantle epithelium. Near the testis it is highly convoluted and functions as a seminal vesicle for the storage of sperm (Fig 4).
Figure 3. The right side of a female Ilyanassa obsoleta in reproductive condition (Redrawn from Jenner, 1978). Gastrop148La.gif
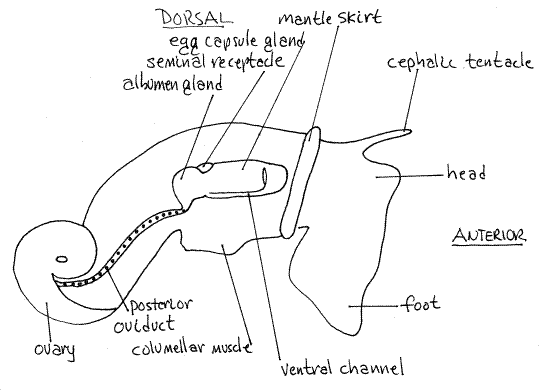
Running anteriorly from the seminal vesicle is the vas deferens which may also be convoluted. The seminal vesicle and vas deferens in this region are both red in mature males but in the reproductive season the sperm in them may make them appear glossy white. In the region of the columellar muscle the duct is swollen to form a golden brown prostate gland.
Anterior to the prostate, the duct becomes the pallial duct derived from mantle epithelium (Fig 4). This duct turns ventrally, runs across the floor of the mantle cavity and right side of the head to the penis. This part of the duct may be difficult to see. It runs into the base of the penis and passes internally through this organ to open at its tip. Distally the penis is flattened and hyaline and the duct may be seen passing through it.
As in females, the reproductive system regresses at the end of the reproductive season. The testis shrinks and looses its red-orange color. The seminal vesicle diminishes in size and turns reddish-brown while the vas deferens becomes a thin dark red line. The penis autotomizes leaving a small yellow bump. As in females, parasitism by trematode flatworms may result in abnormalities of the reproductive system.
Reproduction
Mud snail reproductive systems are reduced and inactive from about June to September in North Carolina. Males achieve reproductive condition before females and retain it longer. About November-December both sexes have well developed reproductive systems. Most breeding occurs in February to May and the reproductive systems begin to regress after then.
Figure 4. The right side of a male Ilyanassa obsoleta in reproductive condition (redrawn from Jenner, 1978). Gastrop149La.gif
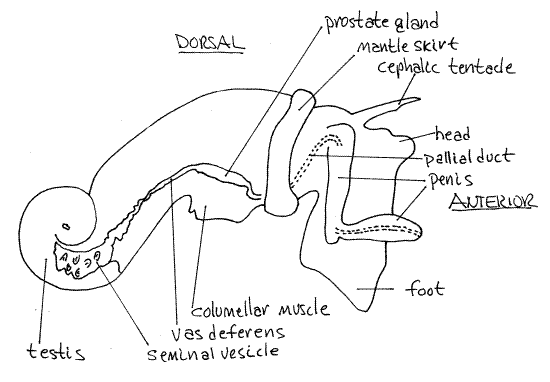
During copulation the male inserts the penis into the female gonopore and deposits sperm. Eggs from the ovary move through the posterior oviduct to the albumen gland where they receive a coating of albumen. They are probably fertilized at about this time also. The exact site of fertilization is not known but is presumed to be upstream of the albumen gland and definitely upstream of the egg capsule gland. Fertilized eggs pass through the egg capsule gland where groups of several eggs are together invested with a soft, malleable, protein coating that will become the egg capsule. This mass of eggs with its protein coating passes through the distal oviduct to the female gonopore and then moves to the pedal gland where the still soft capsule is molded into the species-specific shape and attached to the substratum where it hardens.
Eggs develop in the capsule and emerge as veliger larvae. The planktotrophic veligers eventually settle to the bottom and begin a sedentary omnivorous existence on a mud flat.
Specimens infected by trematodes may have reproductive systems that do not fit the typical pattern. Sometimes parasites may cause the entire reproductive system to atrophy and disappear. At other times the trematodes have no appreciable effect on the reproductive system. This is true of both males and females.
Veliger Larva
Ilyanassa completes early embryonic development in the egg capsule and emerges as planktotrophic veliger larvae. If Ilyanassa have been maintained in a laboratory aquarium, egg capsules and larvae may be present, especially in winter. Egg capsules of this species are bristly, 3 mm tall, transparent, , and attached to hard substrata, including the glass of an aquarium. Each capsule contains several eggs.
>1f. Examine the walls of the aquarium for such capsules and remove one with a razor blade. Place it in a small culture dish of seawater and examine it with the dissecting microscope. Notice its characteristic shape. With the highest power examine the contents of the capsule. It may contain eggs, embryos, or veliger larvae, or it may be empty if the larvae have emerged. If it is empty, find another. Open a capsule with your fine dissecting tools, place its contents in a depression slide and study the developmental stages with the compound microscope. <
>1g. Get a culture dish of aquarium water, examine it for veligers with the dissecting microscope, and make a wet mount if you find one (Fig 12-60A,B). Use wax feet to support the coverslip. Examine the larva with the compound microscope at 100X. Find the coiled shell, known as the protoconch in veligers. The protoconch grows to become the adult shell as the mantle skirt deposits new shell material around the aperture. The ciliated bilobed velum is the larval swimming organ and is not present in adults. When swimming, the velum extends from the aperture into the water. The foot also extends from the aperture and has a tiny operculum attached to its dorsal posterior surface. Some details of the gut may be visible through the transparent protoconch. The style sac, with its intense ciliary activity, is easily observed. <
Internal Anatomy
Digestive System
Relocate the proboscis pore, a small opening between the bases of the tentacles. It opens to the proboscis sheath which contains the extendable proboscis (Fig 2, 12-14B, 12-44). The mouth is located at the tip of the proboscis. Insert fine forceps into the proboscis pore, grasp the proboscis, and pull it out of the proboscis pore so the proboscis is extended as in Figure 2.
" Use fine scissors to open the head with a longitudinal, mid-dorsal incision through the floor of the mantle cavity beginning between the two cephalic tentacles. Remember that the floor of the mantle cavity is part of the dorsal body wall and that it is also the roof of the hemocoel. Accordingly, this incision will open the hemocoel. Extend the incision posteriorly along the mantle cavity well into the visceral mass and anteriorly along the proboscis to the mouth.
In the proboscis, the mouth opens into the short buccal cavity, which contains the radula. Posterior to the radula the gut tube in the proboscis is the anterior esophagus (Fig 2, 12-53, 12-44), which extends for the rest of the length of the proboscis.
>1h. Remove the radula and prepare a wholemount of it being careful that its dorsal surface is up on the slide. Examine the preparation with the compound microscope. Count the number of teeth in each transverse row. Neogastropods have rachiglossate radulae with three teeth per row whereas mesogastropods, with taenioglossate radulae, have seven (Fig 12-40D,C). A rachiglossate radula has a median tooth with a lateral tooth on each side. These teeth form three longitudinal rows that extend the length of the radula. <
The esophagus is very long and extends from the buccal cavity posteriorly through the proboscis, across the floor of the mantle cavity, and into the visceral mass to the stomach, at which point the gut reverses its direction and turns anteriorly again (Fig 12-45B).
Look at the anterior region of the esophagus and find the dark reddish-brown salivary glands located in the floor of the anterior mantle cavity (Fig 2). They secrete mucus and connect via long salivary ducts with the lumen of the buccal cavity far anterior in the proboscis (Fig 12-39A).
A single elongate pale tan or brown Leiblein's gland is located in the vicinity of the salivary glands (Fig 2). It secretes hydrolytic enzymes into the esophagus.
The stomach is a large elongate sac that spirals within the visceral mass. It is closely surrounded by two large digestive ceca except at its left dorso-lateral surface. Digestive ceca are diverticula of the stomach and are connected to it by ducts. The stomach is divided into a pleated posterior gastric cecum and an anterior style sac (Fig 2, 12-39A).
The conical gastric cecum is the posteriormost region of the gut. Torsion has twisted the gut so the esophagus enters the stomach posteriorly and the intestine exits it anteriorly. Consequently, the esophagus empties into the stomach near the gastric cecum. The style sac is anterior to the gastric cecum and opens into the intestine (Fig 12-39A).
>1i. Open the style sac and look for the gelatinous, rod-shaped crystalline style within. It may not be present in laboratory populations maintained on protein diets or in starved animals.<
Two digestive ceca, which have already been noted, open from the stomach and constitute most of the mass of the viscera. The intestine extends from the style sac anteriorly in a sigmoid curve to the anus located atop the large anal papilla in the right mantle cavity. The curved portion of the intestine passes just posterior to the kidney.
Excretory, Hemal, and Nervous Systems
The study of these systems is impractical in these small snails.
References
Brown SC. 1969. The structure and function of the digestive system of the mud snail, Nassarius obsoletus (Say). Malacologia 9(2):447-500.
Jenner MG. 1978. Pseudohermaphroditism: a newly recognized phenomenon in Ilyanassa obsoleta and other Neogastropoda. Ph.D. diss, Dept. Zoology, Univ. North Carolina, Chapel Hill, 168 p.
Ruppert EE, Fox RS. 1988. Seashore animals of the southeast. Univ. South Carolina Press, Columbia.
Ruppert EE, Fox RS, Barnes RB. 2004. Invertebrate Zoology, A functional evolutionary approach, 7 th ed. Brooks Cole Thomson, Belmont CA. 963 pp.
Supplies
Dissecting microscope
Compound microscope
Mud snail
Small dissecting pan
Small vise or 4-inch C-clamp
Isotonic magnesium chloride
Seawater
Methylene blue or other dye in seawater
12-cm Carolina culture dish
Glass slides and coverslips
Small stiff brush (toothbrush)
Small rubber bands
Frozen shrimp