Invertebrate Anatomy OnLine
Dermacentor variablis ©
Dog Tick
29may2007
Copyright 2001 by
Richard Fox
Preface
This is an exercise from Invertebrate Anatomy OnLine , an Internet laboratory manual for courses in Invertebrate Zoology. Additional exercises are can be accessed through the ling on the left. A glossary and chapters on supplies and laboratory techniques are also available through this link. Terminology and phylogeny used in these exercises correspond to usage in the Invertebrate Zoology textbook by Ruppert, Fox, and Barnes (2004). Hyphenated figure callouts refer to figures in the textbook. Callouts that are not hyphenated refer to figures embedded in the exercise. The glossary includes terms from this textbook as well as the laboratory exercises.
Systematics
Arthropoda P, Chelicerata sP, Euchelicerata, Arachnida C, Lipoctena, Apulmonata, Holotrachea, Cryptoperculata, Acarinomorpha, Acari O, Parasitiformes sO, Ixodida, Ixodidae F (Fig 16-15, 18-44, 18-47)
Arthropoda P
Arthropoda, by far the largest and most diverse animal taxon, includes chelicerates, insects, myriapods, and crustaceans as well as many extinct taxa such as Trilobitomorpha. The segmented body primitively bears a pair of jointed appendages on each segment. The epidermis secretes a complex cuticular exoskeleton which must be molted to permit increase in size. Extant arthropods exhibit regional specialization in the structure and function of segments and appendages but the ancestor probably had similar appendages on all segments. The body is typically divided into a head and trunk, of which the trunk is often further divided into thorax and abdomen.
The gut consists of foregut, midgut, and hindgut and extends the length of the body from anterior mouth to posterior anus. Foregut and hindgut are epidermal invaginations, being derived from the embryonic stomodeum and proctodeum respectively, and are lined by cuticle, as are all epidermal surfaces of arthropods. The midgut is endodermal and is responsible for most enzyme secretion, hydrolysis, and absorption.
The coelom is reduced to small spaces associated with the gonads and kidney. The functional body cavity is a spacious hemocoel divided by a horizontal diaphragm into a dorsal pericardial sinus and a much larger perivisceral sinus. Sometimes there is a small ventral perineural sinus surrounding the ventral nerve cord.
The hemal system includes a dorsal, contractile, tubular, ostiate heart that pumps blood to the hemocoel. Excretory organs vary with taxon and include Malpighian tubules, saccate nephridia, and nephrocytes. Respiratory organs also vary with taxon and include many types of gills, book lungs, and tracheae.
The nervous system consists of a dorsal, anterior brain of two or three pairs of ganglia, circumenteric connectives, and a paired ventral nerve cord with segmental ganglia and segmental peripheral nerves. Various degrees of condensation and cephalization are found in different taxa.
Development is derived with centrolecithal eggs and superficial cleavage. There is frequently a larva although development is direct in many. Juveniles pass through a series of instars separated by molts until reaching the adult size and reproductive condition. At this time molting and growth may cease or continue, depending on taxon.
Chelicerata sP
Chelicerata is a large taxon that includes spiders, scorpions, pseudoscorpions, ticks, mites, horseshoe crabs, sea spiders, and many others. The group originated in marine habitats but almost all modern chelicerates are terrestrial.
The body is divided into an anterior cephalothorax with six pairs of appendages and a posterior abdomen which, in most groups, does not bear appendages or has highly modified appendages. The first appendages of the cephalothorax are the chelicerae. Antennae are not present and the brain has two regions rather than the three found in mandibulates. Appendages are primitively biramous but are uniramous in almost all Recent taxa.
Euchelicerata
The segments of the cephalothorax are fused and covered by a dorsal shield, or carapace. Two median eyes are present.
Arachnida C
Arachnids are the terrestrial descendents of the early aquatic chelicerates. The taxon includes the mites, scorpions, pseudoscorpions, spiders, harvestmen, and several other taxa. All are adapted for a terrestrial existence with internal gas exchange and a waterproof integument. Some are capable of silk production and many use toxins to subdue or kill the prey. Most are carnivores, digestion is usually outside the body, and food is liquefied before ingestion.
Acari O
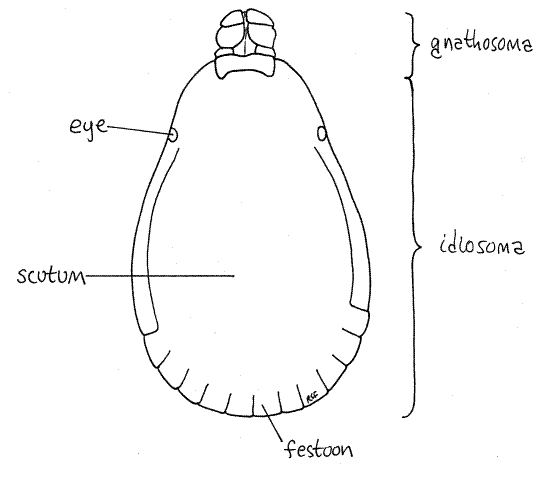
Acari is one of the most successful arachnid taxa with 40,000 known Recent species of ticks and mites. Segmentation is suppressed in acarines and tagmatization reduced to two unique tagmata with no distinction between the more conventional cephalothorax and abdomen. By far the larger tagma is the posterior idiosoma consisting of the old abdomen and most of the cephalothorax. The much smaller gnathosoma (= capitulum) is derived from the anteriormost region of the old cephalothorax. It consists of the chelicerae, pedipalps, preoral cavity, and some of the anterior exoskeleton. The idiosoma bears the four pairs of legs typical of the chelicerate cephalothorax. The gonopore is ventral, on the midline between the posterior pair of legs.
Acarine feeding habits are enormously diverse and they may be herbivorous, carnivorous, or parasitic. Like other arachnids, most liquefy the food externally and ingest it as a liquid. Ticks parasitize terrestrial amniotic vertebrates and many are important disease vectors. The hemal system is reduced and usually lack a heart. Tracheae are present in most. Excretion is accomplished by a pair of saccate nephridia and 1-2 pairs of Malpighian tubules. The central nervous system is highly cephalized and all former segmental ganglia are coalesced into the subesophageal ganglion. Acarines are gonochoric and fertilization is internal, although sperm transmission is usually indirect with spermatophores. The life cycle of most acarines, but not hard ticks, includes a larval and three nymphal instars between egg and adult. Larvae have three pairs of legs, nymphs and adults have four.
Parasitiformes sO
Parasitiformes (= Anactinotrichida) is one of three higher acarine taxa. It includes about 75 families of ticks (Ixodida) and related mites (Mesostigmata, Holothyrida). Parasitiform setae lack actinochitin in contrast with those of mites. The anus is covered by sclerites and trichobothria are absent. The gnathosoma is encircled by a cuticularized ring.
Ixodida
Ticks are larger than mites (2-30 mm), have a leathery integument, a relatively larger gnathosoma, and toothed chelicerae. The first tarsus has a sensory pit known as Haller’s organ. Ticks are obligatory blood-sucking parasites that spend only most of their life off the host. The tick leaves the host following the meal and most of its life is spent off the host. Hard ticks require one blood meal during each instar in order to molt to the next instar. Each instar usually feeds on a different host species. Adult females must have a blood meal to mate and produce eggs and may consume 50 times their weight in blood. Adult males of some species do not feed. Soft ticks are similar but may have more than one blood meal per instar. Ixodida is divided into two noteworthy taxa (and a third with only one species).
Argasidae, the soft ticks, have no scutum and a soft, leathery cuticle. Both sexes become distended when engorged with blood. The gnathosoma is ventral and is not visible in dorsal view. Soft ticks exhibit no sexual dimorphism. About 140 species are known from reptile, bird, and mammal hosts but most are found on birds. Argas and Ornithodoros are the only large taxa.
Ixodidae, the hard ticks, have a stiff dorsal integument, the scutum. Only females become distended when engorged. The gnathosoma is clearly visible in dorsal view. Hard ticks are sexually dimorphic, the sexes differing most notably in the size of the scutum. The basic acarine life cycle is modified in hard ticks so it consists of egg, larva, nymph, adult, with one, rather than three, nymphal instars. Hard ticks are chiefly parasites of mammals. Most ticks, about 650 species, belong to this taxon. Some more common North American genera are Dermacentor, Ixodes, Rhipicephalus, and Amblyomma.
Laboratory Specimens
Ixodid ticks of several genera can be collected from a variety of mammalian hosts of which dogs are probably the most convenient. This exercise is written specifically for the dog tick,Dermacentor variabilis but can be used with other ixodid (hard) ticks. Dermacentor is ornate (with silver or white dorsal markings) and has 11 festoons and a pair of eyes. NoteworthyDermacentor species are D. variabilis (American dog tick) and D. andersoni (Rocky Mountain wood tick, which is the vector for Rocky Mountain spotted fever). Ixodes, the largest tick genus, lacks festoons, is inornate, and has no eyes. The anal groove encircles the anus anteriorly, rather than in its more usual posterior location. A few of its 40 species are I. scapularis (deer tick, the vector of Lyme disease),I. pacificus (on deer, cattle, and dogs in California), I. ricinus (European castor bean tick, named for its castor bean shape and markings). Rhipicephalus is inornate with eyes and festoons. Rhipicephalus sanguineus, the brown tick, occurs worldwide. Amblyomma includes about 100 species of ornate, eyed ticks with festoons. Amblyomma americanais the Lone Star tick found in the southern US and Central America on a variety of hosts, including humans.
Figure 1. Dorsal view of a male Dermacentor variabilis from Whittier, North Carolina. Legs have been omitted. Acarina50L.gif
Ticks are easiest to manipulate if preserved in 40% isopropyl alcohol. Preservation is not necessary, however, and specimens can be observed alive if desired. Students with access to hosts can provide their own ticks. The study should be conducted with a dissecting microscope with the specimens immersed in water or alcohol in a 6-cm culture dish. This exercise is limited to external anatomy. If possible each student should have male, unengorged female, and engorged female ticks. At a minimum each student should have either a male or a female unengorged tick. Sexes are easily distinguished. Males have a large inflexible dorsal shield (= scutum) over the anterior end of the body (Fig 1) whereas the scutum of females is much smaller (Fig 4) leaving the posterior end of the body flexible and distensible to accommodate a large blood meal. The author is grateful to Taylor and Jake for cheerfully donating the specimens on which this account is based.
External Anatomy
Tagmata
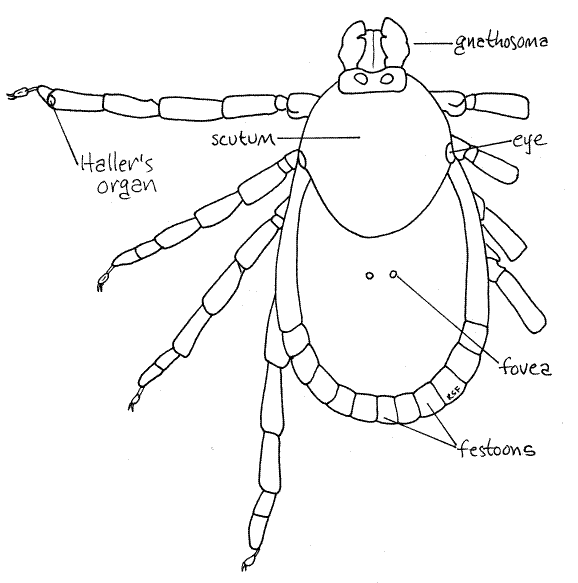
Study a mature, unengorged tick with the dissecting microscope. Examine the dorsal surface. Acarines have suppressed the usual chelicerate tagmatization (cephalothorax and abdomen) and instead the body is divided into a very large idiosoma, which bears four pairs of legs, and a much smaller anterior gnathosoma (= capitulum), which bears the mouthparts (Fig 1, 18-40A). The idiosoma is derived from the abdomen and posterior cephalothorax of the arachnid ancestor and accordingly bears the walking legs and contains the brain. The gnathosoma is the extreme anterior region of the cephalothorax and bears the chelicerae and pedipalps. The gnathosoma is not identical to the head, rather is only the anterior part of the head.
Gnathosoma
The hard tick gnathosoma is distinct and readily visible both dorsally and ventrally. It is a complex structure but its chief features are relatively simple, although small and not always readily visible (Fig 1, 18-40A).
If your specimen was torn abruptly from its host there is likely to be host skin still caught in the gnathosoma and it must be removed. Use fine forceps to extract it. As you will soon see, the gnathosoma is equipped with recurved (posteriorly pointing) teeth designed specifically to prevent what you are attempting to do, i.e. separate the tick from the host's skin. Remember that the teeth face posteriorly and move the skin in that direction to unhook it from the teeth. Then pull the skin anteriorly to remove it. Repeat the process as often as necessary to free the skin. Now use microneedles to clean away any remaining skin particles that obscure your view.
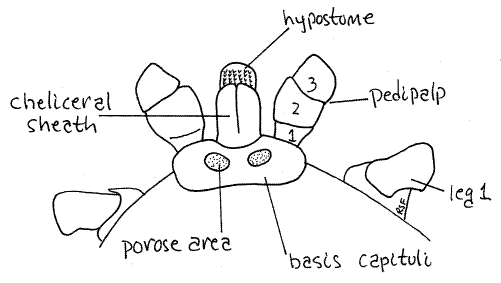
The relevant features of the gnathosoma are the basis capituli, chelicerae, pedipalps, and hypostome. The ringlike basis capituli is the proximal base of the gnathosoma from which the other parts arise (Fig 2). It fits into a notch on the anterior margin of the idiosoma. Protruding anteriorly from the basis, the chelicerae are dorsal, the pedipalps lateral, and the hypostome ventral. They enclose the preoral cavity and the mouth opens between the ventral hypostome and dorsal chelicerae. The pedipalps are the sides of the preoral cavity. The pedipalps curve over the chelicerae dorsally and make viewing the chelicerae difficult. They also partially obscure the hypostome ventrally. Use fine forceps, microneedles and 45X to examine the mouthparts.
Look at the gnathosoma in dorsal view first and find the dorsal part of the basis capituli, (= tectum, tegulum) (Fig 2). The pedipalps extend anteriorly from the sides of the basis and enclose the preoral cavity, chelicerae, and hypostome. The pedipalps are composed of four articles, the proximal trochanter, femur, patella, and small distal tibiotarsus, which can be protracted. On the dorsal midline the basis capituli extends anteriorly to form a tubular cheliceral sheath that encloses the long slender chelicerae. The sheath is visible dorsally but will be mostly covered by the pedipalps until you move them (the pedipalps) aside with your fine forceps or microneedles . Once the pedipalps are out of the way the cheliceral sheath is clearly visible and you can also see part of the toothed hypostome protruding anteriorly beyond the cheliceral sheath.
The chelicerae themselves are not visible, being hidden inside the sheath. They are triarticulate with two articles forming a distal pair of tiny toothed bladelike fingers (Fig 18-43B). The toothed edges of both fingers face laterally and are not chelate as they are in most arachnids. Instead they are tiny blades used to cut into the host's integument, this being their only function. The chelicerae are inactive after penetration is achieved.
Two distinct circular porose areas of the cuticle are evident on the dorsal basis capituli (Fig 2). The porose areas are, like the fovea, perforated cuticular plates through which dermal glands release their secretions.
Figure 2. Dorsal view of the gnathosoma of a female Dermacentor variabilis with the pedipalps moved laterally to reveal the cheliceral sheath. Acarina51L.gif
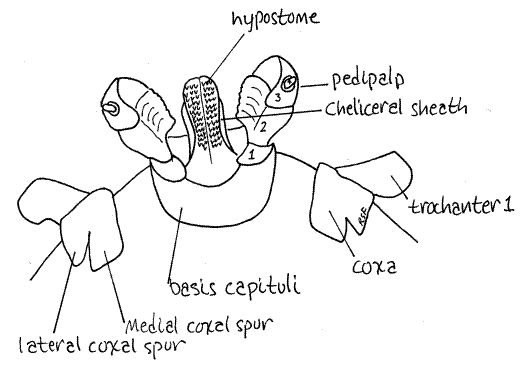
Turn the tick over and look at the ventral gnathosoma. Once again the pedipalps dominate the view but the hypostome can be seen as an anterior projection of the basis capituli (Fig 3). Move the pedipalps laterally to get a better view of the hypostome. The hypostome has six rows of readily visible recurved teeth on its ventral surface. With these teeth the hypostome is an effective holdfast to anchor the tick in the host's skin. It is not involved in cutting into the skin.
Idiosoma
The dorsum of the idiosoma is partially or completely covered by the tough, inelastic dorsal shield, or scutum. In females the scutum is relatively small and is restricted to the anterior end of the idiosoma (Fig 4, 18-40A) whereas in males it covers the entire dorsum of the idiosoma (Fig 1). The large inflexible scutum of males prevents the idiosoma from distending when the gut is engorged. Consequently males consume less than females and do not become distended. Females, with only a small part of the body covered by the inelastic scutum, can become grossly distended when engorged (Fig 6). Any swollen individuals among your specimens are female. The scutum of Dermacentor is ornate, with silver markings.
The posterior margin of the idiosoma of most hard ticks is scalloped with 11 rectangular festoons (Fig 1, 4, 18-40A) (Ixodes has no festoons). A pair of eyes is present on the lateral edge of the scutum (Fig 1, 4). Two inconspicuous, circular, fovea are found beside the midline in about the middle of the dorsum (Fig 4). Fovea are porous cuticular plates through which open secretory foveal glands. Fovea are absent in male Dermacentor variabilis.
Figure 3. Ventral view of the gnathosoma of a female Dermacentor variabilis with the pedipalps moved aside. Acarina52L.gif
The venter of the idiosoma bears the coxae of the four pairs of legs, the gonopore, anus, and spiracles (Fig 5). In both sexes, the gonopore (= genital aperture) is located on the ventral midline between the coxae of the second pair of legs. It is surrounded by a lightly sclerotized genital plate and is at the apex of a long triangular suture, the genital groove, whose base is at the posterior margin of the idiosoma. The anus is also on the ventral midline but farther posterior, although well anterior to the posterior end. It too is surrounded by a sclerite, this one the anal plate. The anal groove is a semicircular suture posterior to the anus. (The anal groove is anterior to the anus in Ixodes.) The two spiracles are lateral, at about the level of the anus just posterior to the fourth legs (Fig 5). Each spiracle consists of a curved slit, the ostium, surrounded by a large oval spiracular plate. The spiracular plate is perforated by numerous pores. The pores and ostium open into a subostial chamber and atrium from which tracheae branch to supply the body with oxygen. Apparently the ventilating current passes through the pores, rather than the ostium. Water loss from expired air is less through the pores than through the ostium. Larval ticks have no spiracles.
Figure 4. Dorsal view of an unfed female Dermacentor variabilis. The right legs have been omitted. Acarina53L.gif
Legs
Study the four pairs of walking legs in ventral view. Each leg consists of a linear series of articles which are, in order from proximal to distal, coxa, trochanter 1, trochanter 2, femur, patella (= genu), tibia, tarsus (with two parts), and the pretarsus (Fig 5). The platelike coxae are embedded in the ventral exoskeleton where they form two longitudinal lateral rows of dark sclerotized plates surrounded by the pale, less sclerotized ventral cuticle (Fig 3). The pale median area is wider in females than in males. The first coxa has two large spurs, one lateral and one medial. The remaining coxae also have two spurs but they are much smaller. The lateral spurs are acute and pointed whereas the medial spurs are short, broad, and rounded apically.
Figure 5. Ventral view of an unfed female Dermacentor variabilis. The left legs have been omitted. Acarina54L.gif
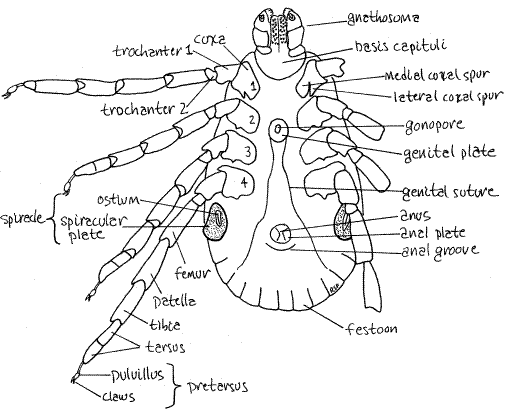
The tarsus, is divided into two subarticles, or tarsomeres. The pretarsus, or foot, is pale, delicate and relatively unsclerotized. Its structure is complex and includes two claws and an adhesive footpad, or pulvillus (Fig 5). Haller's organ is a sensory pit located on the dorsum of the first tarsomere of the first leg at the junction with the second tarsomere (Fig 4). It is small, but readily discernable with high power of the dissecting microscope. The first leg has a sensory function and is used to find and recognize an appropriate host.
Figure 6. A distended female Dermacentor variabilis. Acarina55L.gif
References
Anon. 1967. Pictorial keys to arthropods, reptiles, birds, and mammals of public health significance. U.S. Dept. Health, Education, Welfare, Public Health Service, National Communicable Disease Center. 192 pp.
Baker EW, Wharton GW. 1952. An introduction to Acarology. Macmillan, New York.
Bullough WS. 1958. Practical invertebrate anatomy, 2 nd ed. MacMillan, London. 483pp.
Cheng TC. 1973. General parasitology. Academic Press, New York. 965 pp.
Coons LB, Alberti G. 1999. Acari: Ticks, pp. 267-514 in Harrison FW, Foelix RW. (eds.) Microscopical anatomy of invertebrates, vol 8B, Chelicerate Arthropoda. Wiley-Liss, New York. 514 pp, + index.
Furman DP, Catts EP. 1970. Manual of medical entomology, 3 rd ed. National Press Book, Palo Alto, CA. 163 pp.
Savory TH. 1977. Arachnida 2 nd ed. Academic Press, New York. 340p.
Snodgrass RE. 1971. A textbook of arthropod anatomy. Hafner Publishing Co, New York. 363 pp.
Sonenshine DE . 1991. Biology of ticks, vol 1. Oxford Univ. Press, Oxford. 447 pp.
Sonenshine DE . 1993. Biology of ticks, vol 2. Oxford Univ. Press, Oxford. 465 pp.
Ruppert EE, Fox RS, Barnes RB. 2004. Invertebrate Zoology, A functional evolutionary approach, 7 th ed. Brooks Cole Thomson, Belmont CA. 963 pp.
Warburton C. 1909. Arachnida, in Harmer, S.F. and A.E. Shipley eds, Cambridge Natural History vol IV Crustacea and Arachnids. MacMillan, London.
Supplies
Dissecting microscope
Preserved ticks; male, unfed female, fed female
40% isopropyl alcohol
6-cm culture dish
Microdissecting forceps
Microneedles on applicator sticks