Invertebrate Anatomy OnLine
Daphnia magna ©
Water Flea
19jun2006
Copyright 2000 by
Richard Fox
Lander University
Preface
This is one of many exercises available from Invertebrate Anatomy OnLine , an Internet laboratory manual for courses in Invertebrate Zoology. Additional exercises can be accessed by clicking on the links to the left. A glossary and chapters on supplies and laboratory techniques are also available. Terminology and phylogeny used in these exercises correspond to usage in the Invertebrate Zoology textbook by Ruppert, Fox, and Barnes (2004). Hyphenated figure callouts refer to figures in the textbook. Callouts that are not hyphenated refer to figures embedded in the exercise. The glossary includes terms from this textbook as well as the laboratory exercises.
Systematics
Arthropoda P, Mandibulata, Crustacea sP, Eucrustacea, Thoracopoda, Phyllopodomorpha, Phyllopoda, Cladocera O, Anomopoda sO, Daphniidae F (Fig 16-15, 19-18, 19-90)
Arthropoda P
Arthropoda, by far the largest and most diverse animal taxon, includes chelicerates, insects, myriapods, and crustaceans as well as many extinct taxa such as Trilobitomorpha. The segmented body primitively bears a pair of jointed appendages on each segment. The epidermis secretes a complex cuticular exoskeleton which must be molted to permit increase in size. Extant arthropods exhibit regional specialization in the structure and function of segments and appendages but the ancestor probably had similar appendages on all segments. The body is typically divided into a head and trunk, of which the trunk is often further divided into thorax and abdomen.
The gut consists of foregut, midgut, and hindgut and extends the length of the body from anterior mouth to posterior anus. Foregut and hindgut are epidermal invaginations, being derived from the embryonic stomodeum and proctodeum respectively, and are lined by cuticle, as are all epidermal surfaces of arthropods. The midgut is endodermal and is responsible for most enzyme secretion, hydrolysis, and absorption.
The coelom is reduced to small spaces associated with the gonads and kidney. The functional body cavity is a spacious hemocoel divided by a horizontal diaphragm into a dorsal pericardial sinus and a much larger perivisceral sinus. Sometimes there is a small ventral perineural sinus surrounding the ventral nerve cord.
The hemal system includes a dorsal, contractile, tubular, ostiate heart that pumps blood to the hemocoel. Excretory organs vary with taxon and include Malpighian tubules, saccate nephridia, and nephrocytes. Respiratory organs also vary with taxon and include many types of gills, book lungs, and tracheae.
The nervous system consists of a dorsal, anterior brain of two or three pairs of ganglia, circumenteric connectives, and a paired ventral nerve cord with segmental ganglia and segmental peripheral nerves. Various degrees of condensation and cephalization are found in different taxa.
Development is derived with centrolecithal eggs and superficial cleavage. There is frequently a larva although development is direct in many. Juveniles pass through a series of instars separated by molts until reaching the adult size and reproductive condition. At this time molting and growth may cease or continue, depending on taxon.
Mandibulata
Mandibulata is the sister taxon of Chelicerata and in contrast has antennae on the first head segment, mandibles on the third, and maxillae on the fourth. The brain is a syncerebrum with three pairs of ganglia rather than the two of chelicerates. The ancestral mandibulate probably had biramous appendages and a J-shaped gut, posterior-facing mouth, and a ventral food groove. The two highest level mandibulate taxa are Crustacea and Tracheata.
Crustacea sP
Crustacea is the sister taxon of Tracheata and is different in having antennae on the second head segment resulting in a total of 2 pairs, which is unique. The original crustacean appendages were biramous but uniramous limbs are common in derived taxa. The original tagmata were head but this has been replaced by head, thorax, and abdomen or cephalothorax and abdomen in many taxa. Excretion is via one, sometimes two, pairs of saccate nephridia and respiration is accomplished by a wide variety of gills, sometimes by the body surface. The nauplius is the earliest hatching stage and the naupliar eye consists of three or four median ocelli.
Eucrustacea
Eucrustacea includes all Recent crustaceans except the remipedes. The taxon is characterized by a primary tagmosis consisting of heat, thorax, and abdomen although the derived condition of cephalothorax and abdomen is more common. Eight is the maximum number of thoracic segments.
Thoracopoda
In the ancestral thoracopod the thoracic appendages were turgor appendages used for suspension feeding in conjunction with a ventral food groove. Such appendages and feeding persist in several Recent taxa but have been modified in many others.
Phyllopodomorpha
The compound eyes are stalked primitively although derived sessile eyes occur in many taxa.
Phyllopoda
Phyllopoda consists of about 800 species in four higher taxa; the “large phyllopodans” consisting of Notostraca, Laevicaudata, and Spinicaudata and Cladocera, which are the “small phyllopodans”. Trunk appendages are phyllopods and a large carapace encloses much or all of the body. Large phyllopodans typically inhabit relictual habits where fishes are absent but Cladocerans show no such restrictions. Tagmata are a head, thorax, and reduced abdomen. The abdomen lacks appendages but has a posterior caudal furca on the telson. A ventral food groove is usually present and employed in feeding. A so-called dorsal organ is present on the dorsal midline of the posterior head.
Cladocera O
The 11 families of Cladocera contain about 800 species of mostly freshwater planktonic and benthic crustaceans. Cladocerans, or water fleas, are small (0.2-6 mm) aquatic crustaceans. Most inhabit quiet fresh waters. Along with the rotifers and copepods they account for most of the freshwater zooplankton.
The carapace in most taxa is large and bivalved so it encloses all of the body except for the head. The two compound eyes are fused on the midline. A naupliar eye is present through life. The body is laterally compressed. The enlarged second antennae are locomotory (swimming) organs but the first antennae are vestigial in females and not much larger in males. The thorax is short with only four to six segments and the abdomen lacks appendages.
Most are suspension feeders that consume phytoplankton which they filter from the water using the seta of the thoracic appendages. A few are carnivores preying on other cladocerans.
Cladocerans are parthenogenetic and for most of the year populations consist entirely of females which reproduce asexually. Males are rarely seen. The carapace encloses a brood pouch in which embryos are retained and direct development occurs. As winter (or sometimes summer) approaches, males appear and sexual reproduction occurs and results in the production of resistant, over-wintering eggs. Such eggs may be enclosed in a purselike ephippium that rests in the sediment at the bottom of the lake or pond until the resting eggs hatch into parthenogenetic females.
Anomopoda sO
Anomopods have a short trunk and large bivalve carapace. The head is expanded dorsally and laterally to form a head shield. Development is direct and resting eggs are enclosed in an ephippium.
Laboratory Specimens
A study of cladoceran anatomy can be based on live specimens collected with plankton tows in local lakes or ponds or on inexpensive cultures available from Carolina Biological or Wards Natural Science. Daphnia magna is well suited for this purpose. It is a very large species, as cladocerans go, and one that is easily maintained in laboratory cultures.
External Anatomy
Place a few Daphnia in an 8-cm culture dish of pondwater and observe the animals using the dissecting microscope. Note the characteristic jerky swimming motion. The uneven appearance of this motion is a result of there being only one pair of locomotory appendages, or oars. Try to observe the movement of the large second antennae, which are the swimming appendages. Observation may be improved by removing most of the water from the dish so that the animals are immobilized in the surface film against the bottom of the dish.
Capture an individual with a large-bore plastic pipet, taking note of the effective evasive action of which these animals are capable. Prepare a wet mount of a single specimen using a coverslip with wax feet. Because of the thickness of these animals they require thick feet to support the coverslip. Place the slide on the stage of the compound microscope and examine it with 40X and 100X as needed. Do not use 400X unless specifically told to do so.
Tagmata
The body of Daphnia is laterally compressed, a condition that is exaggerated by the pressure of the coverslip. The body is divided into an anterior head, a middle thorax, and a posterior abdomen (Fig 1, 19-15A). The thorax and abdomen are enclosed in the carapace but the head is not and extends anteriorly in front of the carapace.
Head
The cladoceran head is bent ventrally and that of Daphnia magna is smoothly rounded. In many species the anterior end of the head is produced into a process or head spine (Fig 19-15B) but it is not in D. magna. The ventral area of the head of Daphnia is extended to form a pointed rostrum (Fig 1). The head is joined to the remainder of the body dorsally but is separated from it ventrally by a deep cleft.
The large biramous second antennae are the most conspicuous feature of the head and one arises on each side near the middle of the head (Fig 1). The second antenna consists of a single basal article, the peduncle, which is controlled by powerful muscles. The muscles are visible through the transparent body wall. Two rami arise from the distal end of the peduncle. In D. magna there are four articles in the upper ramus and the lower ramus has three.
The two rami bear large plumose natatory setae. Look at the setae with higher power and note the rows of tiny pinnately arranged setae on the large setae. The result is featherlike, hence the adjective, plumose, like a plume. The antennae are oars and the setae increase the surface area in contact with the water during the power stroke. During the recovery stroke they collapse and their surface area is reduced.
The female first antenna is very small but you can probably see it just posterior to the tip of the rostrum (Fig 1). It is larger in males where it has a sensory function but it is very unlikely that your specimen is a male.
The mandibles are well developed, although not readily apparent in wholemounts. Each is a long oval with sclerotized teeth at the distal end (Fig 1, 19-15A). The teeth of the right and left mandibles lie on either side of the mouth at the posterior end of the head.
The first maxillae are very small and the second maxillae are absent.
The paired lateral compound eyes of the ancestor (and embryo) are fused on the midline of adults to form a single median compound eye in the interior of the head. It is equipped withocular muscles and you will probably see it move. The muscles extend posteriorly from the eye. You can also see the cluster of small lenses around the periphery of the black pigment in the interior of the eye.
Many cladocerans, including D. magna and D. pulex, have a single, tiny, median, naupliar eye, or ocellus, posterior and ventral to the much larger compound eye. It is embedded in the edge of the brain, which may be visible with careful light adjustment and focusing (Fig 1).
Figure 1 A female Daphnia. Redrawn from Freeman & Bracegirdle (1971). Clad99L.gif
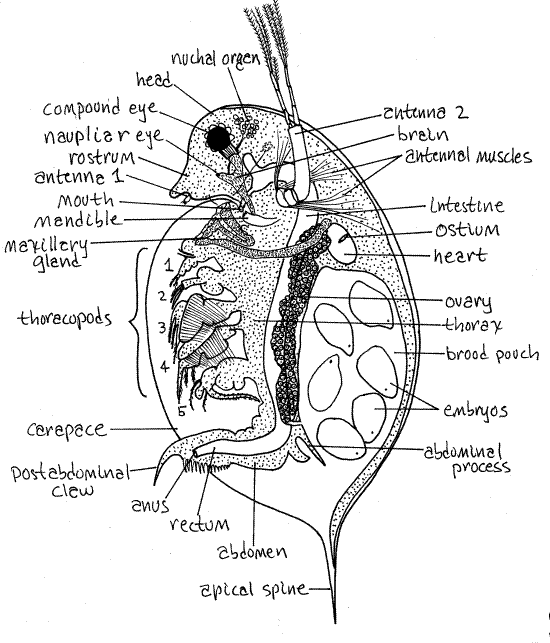
The mouth is located on the ventral head and points posteriorly but you will probably not see it. It lies between the two mandibles.
In some species a shallow notch, the cervical sinus, on the dorsal midline separates the head from the thorax. Daphnia does not have a cervical sinus. Some species have a dorsal organ in the integument of the midline immediately posterior to the cervical sinus but Daphnia does not.
Carapace
The carapace is an enormous double fold of body wall extending posteriorly and laterally from the posteriormost head segment. It has been compared to a cape attached only to the back of the neck. In most cladocerans (anomopods and ctenopods) it a large, thin, flexible sheet folded along the dorsal midline to form two valves, one on either side of the animal. It is laterally compressed. In the few onychopod and haplopod cladocerans it is reduced to a relatively small brood pouch and does not enclose the trunk (Fig 19-16A,B). The carapace is closed dorsally but ventrally and posterior its two valves gape to allow entry and exit of the feeding current (Fig 19-15B).
Many cladocerans, including Daphnia magna, possess a posterior apical spine (= carapace spine) whose function may be to interfere with predation (Fig 1, 19-17B). Many species also have a spine on the head, sometimes a very large one. Planktonic cladocerans are consumed by zooplanktivorous fishes and by other invertebrates, especially the larvae of the phantom midge, Chaoborus.
The carapace is transparent and usually colorless or yellowish. Most of the internal organs, as well as the thoracic appendages are visible through its transparent walls.
Thorax
The thorax is immediately posterior to the head and makes up most of the remainder of the body. It is entirely enclosed in the carapace.
The thorax in Daphnia bears five pairs of biramous, setose thoracopods (= thoracic appendages), the distal tips of which may extend from the ventral gape of the carapace (Fig 1). Four of these appendages generate the feeding current. Their movements draw water from the ventral midline through a setal filter and laterally into the spaces between the limbs from which it exits laterally (Fig 19-12). Food particles are stopped by the setal filter and retained in the ventral food groove in which they move anteriorly from appendage to appendage until they reach the posteriorly directed mouth.
>1a. Place a small dab of petroleum jelly in the center of a dry culture dish. Capture a cladoceran with a large-bore pipet and place it beside the jelly. Use your fine forceps to push the cladoceran upside down into the jelly without damaging it. Do not squeeze it with the forceps. They are absolutely intolerant of being squeezed and it will kill them. You may have to try this with several animals before you are successful.
When you have secured a cladoceran in the jelly, carefully add lake water until the animal is immersed. Observe the upside down creature with 40X of the dissecting microscope (Fig 19-15B). Pay particular attention to the motion of the appendages. Watch the second antennae. Look through the ventral gape of the carapace and watch the thoracopods. Observe the movements of the abdomen. Place a small drop of a carmine suspension in the water and watch the flow pattern of the particles in the swimming and feeding currents. <
Abdomen
Resume study of your wholemount. Posteriorly the thorax narrows to become the abdomen (Fig 1). The abdomen lacks appendages but is flexible, muscular, and highly mobile. It is usually bent sharply forward to tuck beneath the thorax where it is completely enclosed in the carapace.
The anus opens at the posterior tip of the abdomen. A pair of postabdominal claws extends posteriorly from the abdomen. The abdomen and its claws are used to clean the thoracic appendages and remove blockages, such as filamentous algae, to the filter feeding apparatus. A pair of long plumose abdominal setae extends from the dorsal margin of the abdomen.
Two long abdominal processes extend dorsally from the dorsal margin of the abdomen. They function as doors to close the brood pouch and prevent the untimely release of eggs or brooding juveniles. The female lowers the abdominal processes when she wishes to evict her brood from the chamber.
The abdomen can be straightened to extend posteriorly from the carapace. The animal appears to be kicking when it does this and, if your specimen is alive, you will no doubt see it do this.
Hemal System
The hemal system consists of a heart, hemocoel, and blood. The hemocoel is the body cavity. The short oval heart is a conspicuous feature of the dorsal region of the anterior thorax (Fig 1, 19-15A). The heart is surrounded by the hemocoel.
The heart has a single pair of ostia but these may not be evident. No blood vessels are present. Contractions of the heart force blood anteriorly into the hemocoel of the head from which it flows posteriorly into the thorax via three hemocoelic channels. The two lateral channels each serve one side of the carapace whereas the median channel runs ventral to the gut and gives off branches to the thoracic appendages. The carapace is the chief gas exchange surface. Blood returns to the heart from each of these areas.
Digestive System
The C-shaped intestine is easy to see, at least for part of its length, extending from the mouth, through the dorsal thorax, through the abdomen to the anus near the distal end of the abdomen (Fig 1, 19-15A). The regions of the intestine filled with food are easily seen and are likely to be green with phytoplankton or brown with dried cladoceran pellets. Regions that are empty are more difficult to see. A pair of short diverticula, the digestive ceca, arise from the anterior midgut and extend into the head (Fig 1, 19-15A)
Reproductive System
The gonads are long tubes or sacs derived from coelomic spaces extending most of the length of the thorax on either side of the intestine (Fig 1). The gonoducts open to the exterior via gonopores posterior to the last pair of thoracic appendages. In females the ovary opens dorsally, via an oviduct, into the brood chamber. In males the vas deferens leads to a ventral gonopore on the postabdomen.
Female cladocerans have a large water-filled brood pouch located posteriorly under the dorsal carapace (Fig 1, 19-15A). Eggs are extruded here from the oviducts and brooded until they complete embryonic development and become juvenile cladocerans. At this time they are released and begin an independent existence. The brood pouch of your specimen may be filled with eggs, embryos, or juveniles. Try to determine which and look at the specimens of your classmates to see other early life history stages.
Females produce two types of eggs. Summer eggs have little yolk and develop parthenogenetically, without fertilization. Summer eggs, usually numerous, are carried in the brood chamber at least until they hatch and in some species until they are sexually mature and have young of their own.
Resting eggs, on the other hand, are very yolky and thick shelled and are produced only after fertilization. Only two resting eggs are produced, one from each ovary. These eggs are also released into the brood chamber, but are fertilized whereas the summer eggs were not. Winter eggs, unlike those of summer, are not brooded rather are quickly released by the female, either enclosed in a protective cuticular ephippium, or naked, depending on taxon. The ephippium is released when the female molts. Ephippia may sink or float, depending on species. Winter eggs hatch in the following spring. Winter eggs always hatch into parthenogenetic females, i.e. females that reproduce without fertilization. Eventually, after one or several generations of parthenogenetic females and their summer eggs, males are produced and fertilization occurs to produce a new generation of resting eggs.
>1b. If available, look at wholemount slides of Daphnia ephippia (Fig 19-17A). The two eggs in each should be visible with adequate illumination. <
Freshwater Plankton
>1c. A fresh plankton collection from a local lake or pond may be available in the lab. If so, take a small dish of it to your dissecting microscope and examine it. See if you can distinguish between some of the species present. Use a large bore plastic pipet to capture individuals of different species and make wetmounts of them for examination with the compound microscope. The collection will also include copepods and a variety of rotifers. Rotifers are much smaller than cladocerans and copepods and superficially resemble ciliate protozoans. Copepod nauplii will probably be present also (Fig 19-8). <
References
Dodson SI, Frey DG . 1991. Cladocera and other Branchiopoda in Thorp, J.H. & A. P. Covich (eds). Ecology and classification of North American freshwater invertebrates. Academic Press, San Diego.
Freeman WH, Bracegirdle B. 1971. An atlas of invertebrate structure. Hienemann Educational Books, London. 129 pp.
Pennak RW. 1989. Fresh-water invertebrates of the United States, 3 rd ed. Wiley, New York.
Ruppert EE, Fox RS, Barnes RB. 2004. Invertebrate Zoology, A functional evolutionary approach, 7 th ed. Brooks Cole Thomson, Belmont CA. 963 pp.
Supplies
Large bore plastic Pasteur pipet (cut the tip off of a standard plastic Pasteur pipet)
6-cm culture dish
lake water
dissecting kits with beeswax, centimeter rule
dissecting microscopes
compound microscopes
petroleum jelly
Daphnia culture
Wholemount slide of ephippia
carmine suspension