Invertebrate Anatomy OnLine
Corbicula fluminea ©
Asian Clam
13mar2006
Copyright 2001 by
Richard Fox
Lander University
Preface
This is one of many exercises available from Invertebrate Anatomy OnLine , an Internet laboratory manual for courses in Invertebrate Zoology. Additional exercises, a glossary, and chapters on supplies and laboratory techniques are also available at this site. Terminology and phylogeny used in these exercises correspond to usage in the Invertebrate Zoology textbook by Ruppert, Fox, and Barnes (2004). Hyphenated figure callouts refer to figures in the textbook. Callouts that are not hyphenated refer to figures embedded in the exercise. The glossary includes terms from this textbook as well as the laboratory exercises.
Systematics
Mollusca P, Eumollusca, Conchifera, Ganglioneura, Ancyropoda, Bivalvia C, Metabranchia sC, Eulamellibranchia SO, Veneroida O, Corbiculoidea SF, Corbiculidae F (Fig 12-125, 12-122)
Mollusca P
Mollusca, the second largest metazoan taxon, consists of Aplacophora, Polyplacophora, Monoplacophora, Gastropoda, Cephalopoda, Bivalvia, and Scaphopoda. The typical mollusc has a calcareous shell, muscular foot, head with mouth and sense organs, and a visceral mass containing most of the gut, the heart, gonads, and kidney. Dorsally the body wall is the mantle and a fold of this body wall forms and encloses that all important molluscan chamber, the mantle cavity. The mantle cavity is filled with water or air and in it are located the gill(s), anus, nephridiopore(s) and gonopore(s). The coelom is reduced to small spaces including the pericardial cavity containing the heart and the gonocoel containing the gonad.
The well-developed hemal system consists of the heart and vessels leading to a spacious hemocoel in which most of the viscera are located. The kidneys are large metanephridia. The central nervous system is cephalized and tetraneurous. There is a tendency to concentrate ganglia in the circumenteric nerve ring from which arise four major longitudinal nerve cords.
Molluscs may be either gonochoric or hermaphroditic. Spiral cleavage produces a veliger larva in many taxa unless it is suppressed in favor of direct development or another larva. Molluscs arose in the sea and most remain there but molluscs have also colonized freshwater and terrestrial habitats.
Eumollusca
Eumollusca, the sister taxon of Aplacophora, includes all molluscs other than aplacophorans. The eumolluscan gut has digestive ceca which are lacking in aplacophorans, the gut is coiled, and a complex radular musculature is present.
Conchifera
Conchifera, the sister taxon of Polyplacophora, includes all Recent molluscs other than aplacophorans and chitons. The conchiferan shell consists of an outer proteinaceous periostracum underlain by calcareous layers and is a single piece (although in some it may appear to be divided into two valves). The mantle margins are divided into three folds.
Ganglioneura
Most Recent molluscs are ganglioneurans, only the small taxa Aplacophora, Polyplacophora, and Monoplacophora are excluded. Neuron cell bodies are localized in ganglia.
Ancyropoda
The mantle cavity, with its gills, is lateral. The calcareous portion of the shell is bivalve, with the valves opening laterally and joined dorsally by a derivative of the periostracum.
Bivalvia C
Bivalvia is a large, successful, and derived taxon. The body is laterally compressed and enclosed in a bivalve shell. The two valves are hinged dorsally. The the foot is large and adapted for digging in the ancestral condition. A crystalline style is usually present but never is there a radula. The mantle cavity is lateral and in most bivalves the gills are large and function in respiration and filter-feeding. The head is reduced and bears no special sense organs. The nervous system is not cephalized. The group includes scallops, clams, shipworms, coquinas, marine and freshwater mussels, oysters, cockles, zebra mussels, and many, many more.
Metabranchia sC
Metabranch gills are adapted for filter feeding. Water enters the mantle cavity posteriorly.
Eulamellibranchia SO
Eulamellibranchs have gills with tissue interfilamentar connections.
Veneroida O
Shell is usually equivalve and without a nacreous layer.
Laboratory Specimens
Corbicula fluminea is an Asian species that was introduced to the west coast of North America around 1925. Since that time it has spread across the continent and is present in streams, canals, lakes, and reservoirs south of 40 ° North latitude. Its range continues to expand and it can be collected locally for laboratory use in many parts of the United States. It is common in California, western Arizona, parts of Washington and Oregon, throughout the southeast north through Kentucky and sporadically in more northern states. It is absent from most of the Great Plains and Great Basin. Corbicula lives in sand or gravel bottoms with the posterior third of shell exposed above the substratum. It has very short siphons and consequently must live at the sediment surface.
Corbicula is often abundant and population densities can reach 130,000/m 2 but are usually much less, about 10-3000/m 2. It is used as human food in Asia. It is often common in reservoirs where its densities are greatest near the shore.
Corbicula is hermaphroditic, both simultaneous and protandric, has a benthic crawling larva known as a pediveliger which has made it possible for this species to spread rapidly both upstream and downstream in any drainage to which it is introduced. Corbicula competes with native mussel species and is thought to reduce their population densities and may be responsible for the extinction of some species.
Corbicula is recommended as for the study of bivalve anatomy as an alternative to the widely used freshwater mussels. It is a typical eulamellibranch and in many areas it is abundant and readily available, making live dissection feasible at low cost. Furthermore, it is an undesirable alien species. It is preferable to use introduced, overwhelmingly abundant exotic species for study than to sacrifice increasingly scarce native freshwater mussels, several of which are threatened or endangered. Conduct the dissection under magnification in a small dissecting pan immersed in 7% ethanol (if living) or tap water (if preserved).
External Anatomy
Study the external features of an intact clam. The soft parts are completely enclosed in the shell. The shell consists of two valves (Figs 1, 2), right and left. The two valves are held together along their dorsal margins by the hinge. The umbo is a protuberance beside the dorsal margin of each valve. The umbo is displaced slightly toward the anterior end of the valve. Knowing dorsal and anterior find right, left, ventral, and posterior.
Note the shiny brown organic periostracum covering the outside of the valves. Bright white calcareous layers of the shell may also be visible where the periostracum has been eroded, especially near the umbos
" Living Corbicula, like most bivalves, are difficult to anesthetize and open. At their first detection of anything undesirable in their environment the animal “clams up” and refuses to expose itself to an anesthetic. Corbicula is much easier to open than are larger clams such as Mercenaria.
Use the following instructions to open the clam so that the adductor muscles are cut, the right valve is removed, and the clam is left cradled in its left valve with its right surface exposed for observation. Be very careful that the scalpel does not slip and cut you instead of the clam. Refer to Figure 2 or 3 to determine the location of the anterior and posterior adductor muscles. These muscles must be cut before the clam can be opened.
Carefully slip the blade of a scalpel between the valves at the anterior end of the clam. Do not push toward your hand while you do this. Cut through the anterior adductor muscle and then gently push on the scalpel handle so that the tip of the blade is against the inside surface of the right valve. Carefully work the blade around the ventral perimeter of the shell from anterior to posterior so that the blade scrapes the soft tissue away from the right valve. Cut the posterior adductor muscle. You should not cut any of the soft tissue other than the adductor muscles. To that end, be sure to keep the blade against the right valve.
When the two adductor muscles are severed or scraped away from the right valve, gently lift the right valve. Open the shell slightly so you can see inside and use the scalpel to scrape gently (not cut) all remaining soft tissue away from the right valve so it stays with the rest of the animal in the left valve. When all soft tissue is removed from the right valve, remove the valve and set it aside. The complete clam is now present in its left valve with its right surface uppermost and ready for study. The right mantle skirt may have been damaged by the scalpel but the rest of the clam should be intact.
Place the clam in a small dissecting pan of 7% non-denatured ethanol. The clam should be completely immersed in the anesthetic. The right surface of the clam should be up. Once in the anesthetic the clam will begin to relax. Until that time its muscles, including those of the mantle, will be contracted. Set the pan aside. While you wait for the clam to relax, study the anatomy of an empty shell.
Shell
Examine a cleaned shell. It consists of two valves (Fig 1, 2). The umbo is a protuberance beside the dorsal margin of the valve. It is often called the "beak" and is the oldest part of the valve. It makes a good landmark for orienting the clam. It is dorsal and in most bivalves is displaced toward the anterior end of the valve and/or points toward the anterior end. The plane of symmetry passes between the two valves, which are thus right and left. Place the two valves together, orient the animal, find the plane of symmetry and relocate the major directions;dorsal/ventral, anterior/posterior, and right/left.
The two valves of your dried shell are probably no longer connected to each other but in life they would be held together along their dorsal margins by an articulation known as the hinge(Fig 2, 12-92B). The umbos are situated beside the hinge and arch toward it and toward each other.
The hinge region possesses projections of the shell known as hinge teeth and a pad of elastic protein known as the hinge ligament (Fig 2, 12-92B). The teeth are readily visible on the inside of the hinge of each valve and the ligament should also be visible unless it has been broken off by handling. It is a dark brown mass of protein that becomes very brittle in dried specimens. In Corbicula it is external and located immediately posterior to the umbo.
Shell Layers
The typical bivalve shell consists of three layers; the outer periostracum, middle prismatic layer (= ostracum), and the inner lamellar layer (= hypostracum) (Fig 12-91). All three layers are secreted by the mantle epidermis. Corbicula has a well developed and conspicuous periostracum and lamellar layers but the prismatic layer is reduced. Most of the mass of the shell is the lamellar layer.
The periostracum, which in Corbicula is dark olive brown or black, is the outermost layer. It is composed of the protein conchiolin. Inside the periostracum is a chalky white prismatic layer of calcium carbonate crystals deposited over an organic collagenous matrix. The periostracum, in the vicinity of the umbo especially, is often eroded in freshwater clams. Consequently, the underlying white calcareous layer is exposed and visible externally. In areas where the periostracum is missing the underlying calcareous shell is subject to erosion by acidic water so that the shell is often pitted. Innermost is the thick, lamellar layer , which is also calcium carbonate and an organic matrix. It can be seen covering the inside surface of the valves. It is purple and white.
In some molluscs the structure of the lamellar layer is such that its appearance is smooth and lustrous. This type of shell is known as "mother of pearl" or nacre (pronounced NAKE ur). The lamellar layer of freshwater “pearly” mussels (Unionida) is nacreous but that of Corbicula, and other Veneroida, is not.
Figure 1. Exterior of the left valve of the Asiatic clam, Corbicula fluminea. Mussel83La.gif
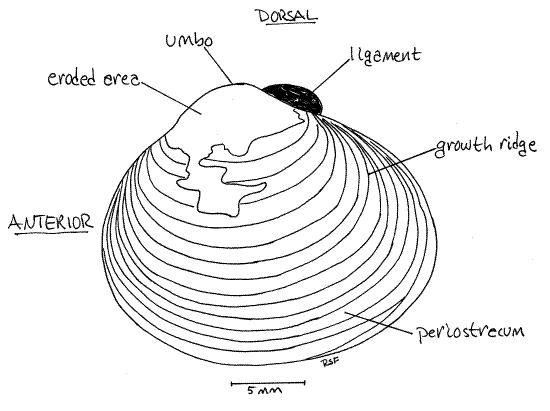
New shell material is deposited by the mantle epidermis along the margins of the valve. Periods of growth are marked by conspicuous concentric growth ridges on the outer surface of the valve (Fig 1).
Hinge
Look at the inside of one of the valves and observe the architecture of the hinge region. Corbicula is a good species for demonstrating the basic pattern of bivalve hinge teeth. The function of the hinge teeth is to keep the valves in alignment. The dentition on the right and left valves differ (because they must mesh with each other) but in Corbicula the differences are slight so it makes no difference which valve you study.
In the center of the hinge, immediately ventral to the umbo, are the cardinal teeth (Fig 2, 12-92B). (In freshwater mussels the teeth in this position are known as pseudocardinal teeth.) In Corbicula there are three cardinal teeth in each valve and they are easily recognized because they look like teeth.
Figure 2. Interior of the right valve of the Asiatic clam, Corbicula fluminea. Mussel84La.gif
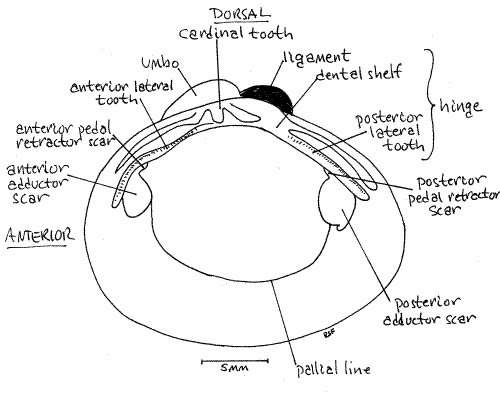
In addition to the cardinal teeth, two lateral teeth are present in the hinge of each valve but they don't look much like teeth. They are low straight ridges paralleling the dorsal edge of the valve. The anterior lateral teeth are anterior to the cardinal teeth and the posterior lateral teeth are posterior to them. The right valve has two of each, whereas the left valve has only one. Look at the two valves and see if you understand the functional reason for this asymmetry.
Fit the two valves together to demonstrate how the teeth mesh together to keep the valves in alignment when closed. Try to shear the two valves past each other while the valves are tightly closed and you will appreciate the effectiveness of the hinge teeth.
The teeth are located on a part of the hinge known as the dental shelf. There is typically a cavity under the dental shelf and inside the umbo known as the beak cavity. Corbicula has a deep beak cavity.
Muscle Scars
In life the two valves are pulled together by a pair of adductor muscles, one anterior and one posterior. These muscles extend transversely across the clam from one valve to the other. When contracted they pull the valves together. This action also stretches the hinge ligament, which is elastic. When the adductor muscles relax the hinge ligament returns to its original shorter length and this pulls the umbos closer together and the ventral margins of the valves move apart. The shell thus opens slightly along the ventral border. The small gap thus created between the edges of the two valves is the gape. It is just wide enough to allow the foot to slip out.
Look again at the inside surface of one of the valves. You will see two smooth elliptical areas, one anterior and one posterior. These are the anterior and posterior adductor muscle scars , respectively, and they are the sites of attachment of the adductor muscles.
Associated with the adductor muscle scars are scars of the pedal retractor muscles that withdraw the foot into the shell before the valves are adducted. These small scars are located on the margins of the adductor scars and often coalesced with them.
The anterior pedal retractor muscle scar is located at about 1:00 on the circumference of the anterior adductor scar. It is hidden by the overhang of the anterior lateral tooth. Theposterior pedal retractor muscle scar is located at about 11:00 on the outline of the posterior adductor scar and is hidden by the posterior lateral tooth.
The pallial line extends from one adductor scar to the other and parallels the ventral border of the valve. This line marks the site of attachment of the mantle and its pallial muscles. The mantle will be considered in more detail later.
Soft Anatomy
Introduction
Turn your attention to the opened, live clam you set aside earlier. It should be anesthetized now and unable to contract. Place the dissecting pan on the stage of the dissecting microscope and examine the animal with low power. If the soft anatomy is intact, you will be looking at the outside surface of the right mantle skirt (Fig 3, 12-90). The right mantle skirt (= mantle lobe) is penetrated by the two adductor muscles. You cut these muscles in order to open the shell but they will still be in place attached to the left valve. Find the anterior adductor muscle ventral to the anterior lateral tooth and the posterior adductor muscle ventral to the posterior lateral tooth (Fig 3, 12-89A).
Mantle and Mantle Cavity
In life the periphery of the right mantle skirt would be attached to the right valve by a sheet of transparent, slightly yellowish periostracum but, since you have removed the valve, that connection has been broken. With magnification, look at the edge of the right mantle skirt to see the remnants of this sheet of periostracum. It looks like plastic wrap or cellophane. The periostracum is secreted by the margin of the mantle and the sheet you see was, before dissection, continuous with the periostracum covering the right valve. Lift the right mantle skirt and find the margin of the left mantle skirt. The periostracum of this skirt should still be intact and connected with the shell. Examine it with the dissecting microscope.
The space between the right and left mantle skirts is the mantle cavity. This space is outside the body and is not a body cavity, even though it is largely enclosed by the shell and mantle. In life it is filled with the water that is the animal's environment. The mantle cavity consists of two parts. The part you see now is its inhalant chamber (= branchial chamber).
Look at the posterior edges of the right and left mantle skirts and see that they are joined with each on the midline to form a pair of openings, the siphons (Fig 3, 8, 12-89). The mantle tissue is thickened in the vicinity of the siphons and is pigmented. The ventral siphon is the inhalant siphon and it is continuous with the inhalant chamber. It is the larger of the two and its external opening is guarded by tentacles of various sizes whose purpose is sensory and mechanical (to exclude large particles). Use a needle or probe to demonstrate the connection between the inhalant siphon and the inhalant chamber.
Figure 3. The right side of Corbicula with the right valve and mantle skirt removed. Mussel85La.gif
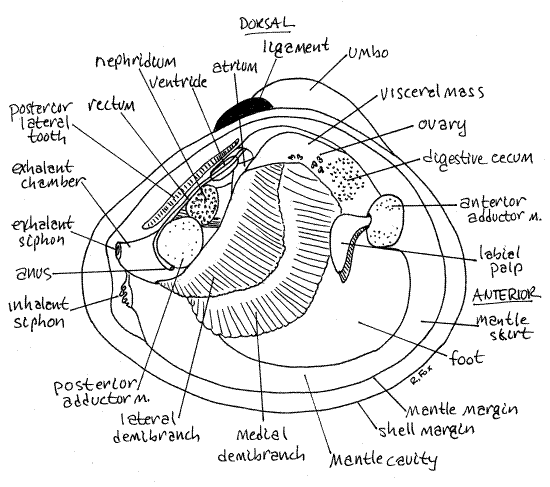
The dorsal opening is the exhalant siphon and it is the smaller of the two siphons (Fig 3, 8, 12-89). It does not possess the array of sensory tentacles found on the inhalant siphon. It is the outlet from the exhalant chamber of the mantle cavity dorsal to the gills. You cannot demonstrate the continuity between the exhalant siphon and the exhalant chamber at this time.
The mantle cavity is divided by the gills into a ventral inhalant chamber (which you have seen) and a dorsal exhalant chamber, or suprabranchial chamber, (which you cannot see yet). Water passes through the inhalant siphon to enter the inhalant chamber, flows across the gills and then into the exhalant chamber. As the water crosses the gills food particles are filtered from it and oxygen is removed. From the exhalant chamber the water flows out the exhalant siphon.
" Use fine scissors to remove the right mantle skirt. Leave the siphons intact but cut around them to remove the rest of the right mantle. The margin of a typical bivalve mantle skirt has three longitudinal folds, each with a specific function (Fig 4, 12-91). The outer fold secretes two of the layers of the shell (periostracum and prismatic layer), the middle fold is sensory, and theinner fold is muscular. The lamellar layer of the shell is secreted by the entire outer surface of the mantle skirt, not by the folds.
Figure 4. Section and oblique view of the shell and mantle margin of Corbicula. Mussel86La.gif
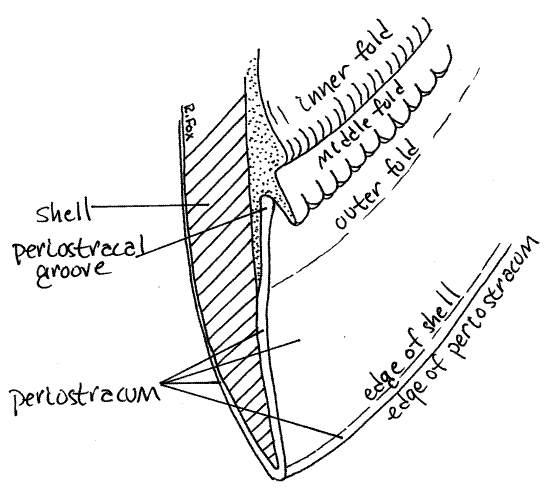
Examine the margin of the left mantle skirt with the dissecting microscope at about 12X. You may also find it instructive to refer back to the edge of the right mantle skirt occasionally. Adjust the light and focus carefully. Find the periostracum emerging from near the edge of the skirt (Fig 4, 12-91). Note that it is attached to the mantle and then extends to the inner edge of the valve and wraps over this edge to continue over the outer surface of the valve. The periostracum is secreted by the inner surface of the outer fold of the mantle margin. It arises in theperiostracal groove between the outer and middle folds.
Gills
Removal of the right mantle exposed the inhalant chamber of the mantle cavity to view and gave you access to the structures in it. You can now see the right gill, foot, visceral mass, and left mantle skirt (Fig 3, 12-89A). The left gill is hidden by the foot and visceral mass. The right gill is a double sheet of corrugated tissue lying on top of the foot and visceral mass (Fig 3). It extends obliquely across the mantle cavity.
The foot is a large semicircular mass of muscle occupying most of the ventral region of the mantle cavity (Fig 3). The size of the foot varies depending on its state of contraction. It is smaller in preserved specimens.
The visceral mass is the thick globular mass of tissue dorsal to the foot and ventral to the hinge (Fig 3, 12-89B). The foot is attached to the ventral border of the visceral mass. The left mantle skirt has already been identified. In dissected specimens contractions of its muscles may pull it away from the margin of the valve.
Look at the right gill. It is a single gill, or holobranch, even though it may appear to be two. It is a typical eulamellibranch gill whose dual purposes are filter feeding and gas exchange. In addition, as is typical of many other freshwater bivalves including the freshwater mussels, part of it serves as a brood chamber for the incubation of eggs.
The gill consists of two sheets of coalesced filaments folded into a "W" shape (in cross section) (Fig 12-90, 12-96C,D). It is attached to the dorsal wall of the mantle cavity by a longitudinal central axis coinciding with the middle point of the "W". This sheet divides the mantle cavity into the ventral inhalant chamber and the dorsal exhalant chamber. To get from the ventral chamber to the dorsal, water must pass through ostia (pores) in the gills.
The holobranch, or whole gill, is composed of two half gills, or demibranchs. Each demibranch is a sheet of fused filaments. Each demibranch corresponds to one of the two "Vs" of which our "W" model is composed. Closest to you is the lateral demibranch of the right gill and under it is the larger medial demibranch (Fig 3). The two are attached to each other and to the mantle along the longitudinal central axis of the gill. They are also attached to the body (either mantle or foot) along their borders. The demibranchs are hollow and the space inside them is the exhalant chamber.
The surface of each side of a demibranch is a lamella. Each demibranch has lateral and medial surfaces, and thus two lamellae. Each holobranch thus has four lamellae. Each demibranch is connected to the central axis by its descending lamella and to the mantle skirt or foot by its ascending lamella (Fig 12-90).
The lamellae are covered by a ciliated epithelium and some of these cilia (the lateral cilia) generate the feeding current that brings water in through the inhalant siphon and then through the ostia into the exhalant chamber and then out the exhalant siphon.
Bivalves feed on suspended particles too large to pass through the ostia and thus are retained on the inhalant side of the gill. Other cilia (the frontal cilia) are responsible for moving these particles, both organic and mineral, over the surfaces of the lamellae and eventually to the labial palps and mouth.
> 1a. If your specimen is alive, remove it from its pan and looking at the gills, with magnification, while they are covered by a thin film of water. Focus on areas where light is reflected from the surface and you will see it shimmer from the activity of the cilia. <
Bivalve gills are composed of numerous long, fused filaments. Look as the surface of a demibranch with about 25X magnification. At this magnification you can easily see the parallelfilaments (Fig 5, 12-96D) of which the lamella, demibranch, and holobranch are composed.
Look at the ventral edge of one of the demibranchs with high power. Here you see the filaments of one lamella bend 180 º and become the filaments of the opposite lamella (Fig 12-90). Along the bend, which is the free edge of the demibranch, there is a distinct ciliated food groove (Fig 5, 12-96B,D). The cilia in this groove create a stream of mucus and food particles that moves anteriorly to the labial palps and ultimately to the mouth.
>1b. If you have a living specimen, place it in a dish of seawater and arrange it in the dish so the flat surface of the exposed lamella is horizontal, or nearly so. Look at the surface of the gill with magnification. Place a little chalk dust or a drop of carmine-seawater suspension on the surface of the gill while watching it with the dissecting microscope. You should be able to see the particles moving rapidly over the gill to the ventral food groove. The particles, and the mucus surrounding them, are moved anteriorly by the ciliary transport mechanism of the food groove. <
Figure 5. A small portion of the food groove on the ventral margin of the medial demibranch of Corbicula. Mussel87La.gif
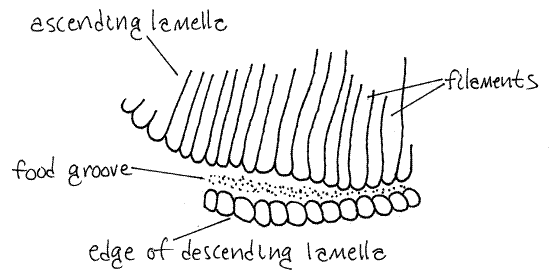
>1c. Remove about 2-3 mm of the edge of a demibranch, place it on a slide, tease the filaments apart, affix a coverslip, and examine it with the compound microscope. If your specimen is alive, the beating cilia of the filament will be easy to see. Look for the ventral food groove at the edge of the gill. Note that it is a deep groove with a narrowed opening formed by the ends of the filaments of the descending and ascending filaments. In living specimens, the beating cilia of the filaments are easily seen. <
In Corbicula, every 16 th filament of the descending lamella is connected by interlamellar junctions to the same filament on the opposite ascending lamella. The other 15 intervening filaments are not connected in this fashion and are free to bulge away from each other. Consequently, the surface of the gill appears corrugated, or plicate. Each ridge is a group of 16 filaments held together only by the 16 th filaments.
" With fine scissors cut along the central axis of the right gill at its posterior end, in the vicinity of the posterior adductor muscle. This will reveal the exhalant chamber inside the gill. Note the vertical water tubes extending into the demibranchs (Fig 12-98C,D). Water from the inhalant chamber passes through ostia in the lamella to enter the water tubes. From the water tubes it moves to the exhalant chamber and exhalant siphon. With a probe, demonstrate the connection between the exhalant chamber and the exhalant siphon.
Labial Palps
Strings of food and mucus are moved anteriorly along the ventral food grooves of the demibranchs to the labial palps at the anterior end of the mantle cavity on either side of the mouth (Fig 3). There is a labial palp on the right of the mouth and another pair on the left. Each pair consists of two triangular sheets of tissue, known as palp lamellae that resemble small gills (Fig 12-100). The outermost is the lateral lamella and hidden under it is the medial lamella.
One surface of each lamella is a sorting field of ciliated ridges and grooves. The other surface is smooth. The ciliated surface of each lamella faces the opposing ciliated surface of the other lamella. More specifically, the medial surface of the lateral lamella faces the lateral surface of the medial lamella. Food and mucus from the food groove of the gill move onto the sorting fields where organic food particles are separated from mineral particles. The food moves along a ciliated oral groove to the mouth, again in a mucus string powered by cilia. The mineral particles, also mixed with mucus, are discarded into the inhalant chamber as pseudofeces. Occasional contractions of the adductor muscles compress the chamber and expel the pseudofeces through the inhalant siphon. Sorting on the gills and labial palps is imperfect and final sorting occurs in the stomach.
The labial lamellae of each side are connected with their counterparts on the opposite side of the anterior visceral mass by a pair of transverse lips. The right and left lateral lamellae are connected by the upper lip and the right and left medial palps are connected by the lower lip (Fig 12-100). The upper lip passes dorsal to the mouth and the lower lip passes ventral to it. The food string travels in the oral groove between the upper and lower lips to reach the mouth.
Hold the clam with one hand so you can see the anterior surface of the visceral mass and the lips with the dissecting microscope. Use a minuten nadel to lift the upper lip so you can see the mouth. The mouth is a tiny, inconspicuous opening located on the midline between the two lips. It can be difficult to demonstrate.
>1d. Separate the two lamellae of the left labial palp as if opening a book. Place a little carmine-seawater on the ridged surfaces of the lamellae and watch it as it is transported by their cilia. Try to trace currents and watch for the development of a stream of particles in the oral groove leading into the mouth. This is probably the easiest way to find the mouth. The mouth is a small and inconspicuous pore but is easy to see if it has a string of red carmine particles entering it. <
Visceral Mass
Spend a moment or two on a superficial preliminary examination of the visceral mass before considering it in more detail. Relocate the visceral mass (Fig 3). It is the large, thick mass of tissue situated immediately ventral to the hinge and occupying the dorsal half of the valve.
The laterally compressed, muscular, cream-colored foot is attached to the ventral edge of the visceral mass. While its appearance is variable, it is likely to be more or less tongue-shaped with the tongue probably pointing anteriorly.
The visceral mass is thickest dorsally and here you may see dark lobes of the digestive ceca through its walls. The color of the digestive ceca depends on the color of the food and may be yellowish, brownish, green, etc.
You may also see parts of the gray nephridium, or kidney. The thin-walled pericardial cavity and the heart are located on the dorsal median edge of the visceral mass immediately ventral to the middle of the posterior lateral tooth (Fig 3, 8). A lobe of the nephridium is present just posterior to the pericardial cavity. The heart is located inside the pericardium.
Although you cannot see it now, the rectum passes through the pericardial cavity, extends posteriorly just ventral to the posterior lateral tooth, between the tooth and the posterior adductor muscle, and then ends at the anus, in the exhalant chamber, on the posterior side of the posterior adductor muscle.
Gonad
Most of the interior of the visceral mass, dorsal mantle skirts, and foot are filled with the gonads. Corbicula is hermaphroditic and there may be testis, ovary, or both present in your specimen. These organs reach the surface of the visceral mass and parts of them are visible without dissection (Fig 3).
Figure 6. The eggs of Corbicula with sperm drawn at the same scale. Mussel88La.gif
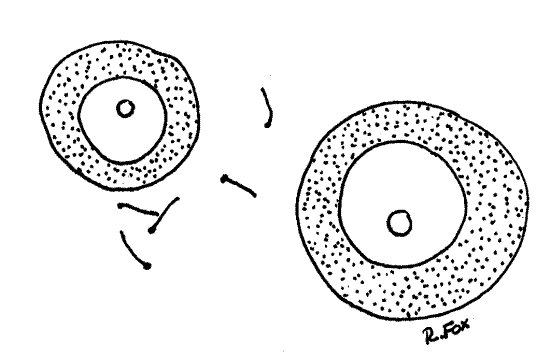
The testis is bright white and the ovary is dark gray. The ovary consists of innumerable fairly large digitiform follicles which contain female sex cells in various stages of development. The follicles are easily seen on the surface of the visceral mass and in the dorsal part of the mantle skirts. Don't confuse the ovary with the nephridium, which is also gray and composed of digitiform processes. The processes of the nephridium are much smaller than those of the ovary.
The testis consists of microscopic seminiferous tubules which contain male sex cells in various stages of development. You cannot see individual tubules with the dissecting microscope.
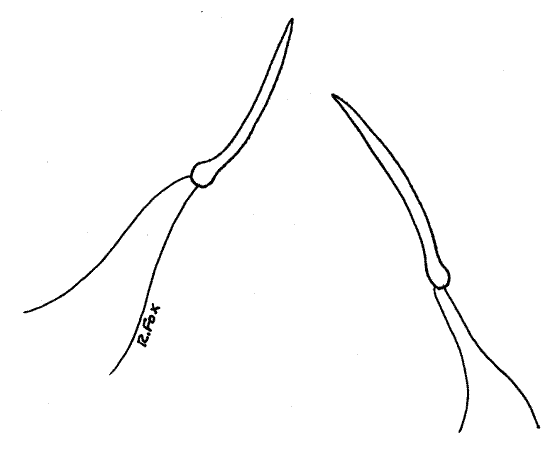
>1e. Make two wet mounts using small bits of ovary for one and testis for the other. Squash the tissue in a drop of water and add a coverslip. Examine the preparation with the compound microscope and look for gametes. Eggs are large nucleate spheres (Fig 6). Sperm are tiny, elongate and biflagellate (Fig 7). <
Sperm are released to the environment in large balls (morulae), each consisting of thousands of sperm, via the exhalant siphon. These enter the inhalant siphon of another clam where the morulae break up into individual sperm which penetrate the gills and enter the exhalant chamber where they fertilize eggs present there. The eggs are then brooded in the interior water tubes of the medial demibranchs.
The larvae (pediveligers) are crawlers, not swimmers, and thus are well adapted for life in flowing water where they can move upstream or downstream along the bottom and avoid being swept downstream as would planktonic larvae. Nor are they dependent on fishes for dispersal as are the parasitic glochidia larvae of freshwater mussels. Larvae are released twice yearly, once in spring and in fall.
Figure 7. The biflagellate sperm of Corbicula. Mussel89La.gif
Pericardial Cavity and Heart
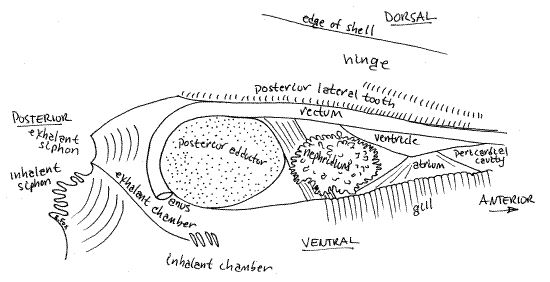
" The heart and pericardial cavity are enclosed in a thin membranous pericardium ventral to the posterior lateral tooth (Fig 3, 8). Use your fine scissors to make a longitudinal incision through the pericardium to open the pericardial cavity. Extend this incision posteriorly around the dorsal edge of the posterior adductor muscle and then to the siphons. Cut away the right side of both siphons. This will expose the rectum, heart, and exhalant chamber (Fig 8).
Find the heart in the pericardial cavity. It consists of a muscular ventricle to which are connected two thin-walled atria, one from each gill. The ventricle has thick, opaque walls and is wrapped around the tubular rectum (Figs 3, 4). The triangular, thin-walled, transparent right atrium extends from the dorsal edge of the right gill to the right side of the ventricle. The atrium may have been damaged or destroyed when you opened the pericardium.
The rectum enters the pericardial cavity anteriorly, passes through the ventricle, and then exits the pericardial cavity posteriorly (Fig 8). It lies immediately ventral to the posterior lateral tooth. It crosses the posterior pedal retractor muscle and then passes over the dorsal surface of the posterior adductor muscle, between the muscle and the lateral tooth, and then curves around the posterior side of the muscle to end at the anus located at about 7:00 on the circumference of the muscle. The anus opens into the exhalant chamber.
Figure 8. Side view from the right of the pericardial cavity, heart, kidney, and exhalant chamber of Corbicula. Mussel90La.gif
References
Britton JC, Morton B. 1982 . A dissection guide, field and laboratory manual for the introduced bivalve Corbicula fluminea. Malacologia Rev. Supp. 3:1-82.
Heard WH. 1968. Mollusca, G1-G26 in F. Parrish (ed), Keys to water quality indicative organisms (Southeastern United States). Federal Water Pollution Control Administration, Washington.
McMahon RF. 2001. Mollusca: Bivalvia, pp.331-430 in Thorp JH, Covich AP (eds.), Ecology and classification of North American freshwater invertebrates, 2 nd ed. Academic Press, San Diego. 1056pp.
Pennak RW. 1989. Fresh-water invertebrates of the United States, 3 rd ed. Wiley, New York.
Ruppert EE, Fox RS, Barnes RB. 2004. Invertebrate Zoology, A functional evolutionary approach, 7 th ed. Brooks Cole Thomson, Belmont CA. 963 pp.
Supplies
Dissecting microscope
Compound microscope
Small dissecting pan
Empty cleaned shell
Living or preserved Corbicula
7% non-denatured ethanol
Carmine-seawater suspension
Corbicula can be collected, sometimes in enormous numbers, along the shallow littoral zone of lakes and reservoirs. They are especially easy to collect when exposed by deliberate water level drawdown in the winter. At other times they can be collected by wading with a shovel and sieve or from a boat or dock using an Ekman dredge or similar sampler. Once collected, specimens can be held in finger bowls, but out of water, in a refrigerator for several weeks. They should be moistened occasionally.
Corbicula can be maintained indefinitely in laboratory aquaria on strained (babyfood) spinach but they will not grow or reproduce on this diet (Britton & Morton, 1982).