Invertebrate Anatomy OnLine
Comactinia echinoptera ©
Feather Star
25may2007
Copyright 2001 by
Richard Fox
Lander University
Preface
This is one of many exercises available from Invertebrate Anatomy OnLine , an Internet laboratory manual for courses in Invertebrate Zoology. Additional exercises, a glossary, and chapters on supplies and laboratory techniques are also available at this site. Terminology and phylogeny used in these exercises correspond to usage in the Invertebrate Zoology textbook by Ruppert, Fox, and Barnes (2004). Hyphenated figure callouts refer to figures in the textbook. Callouts that are not hyphenated refer to figures embedded in the exercise. The glossary includes terms from this textbook as well as the laboratory exercises.
Systematics
Echinodermata P, Crinoidea C, Comatulida O, Comatulacea SF, Comasteridae F (Fig 9-26, 27-12, 28-62)
Echinodermata P
Echinoderms are secondarily radially symmetric deuterostomes whose ancestors were bilaterally symmetric. The adult radial symmetry is pentamerous with body parts occurring in fives or multiples thereof. Echinoderms have strong affinities with the ancestral trimeric deuterostomes especially in the tripartite organization of their coelomic cavities. Echinoderm larvae have the coelom divided into three regions, as is typical of the early coelomates, and these regions have important adult derivatives. All echinoderms are marine and benthic. About 6000 Recent species are known and the fossil record includes 13,000 extinct species.
An important echinoderm apomorphy is the water vascular system that in most taxa functions in support of locomotory tube feet but is also important in gas exchange, excretion, and feeding. The body wall includes a thick connective tissue dermis in which calcareous ossicles (little bones) are almost always an important component. These ossicles make up an endoskeleton that assumes different forms in different taxa. In most echinoderms calcareous spines of various sizes and shapes arise from the dermis and extend from the body surface and are alluded to by the name echinoderm (= spiny skin). The connective tissue is mutable and its consistency is under nervous control.
Excretion in echinoderms is accomplished by simple diffusion of metabolic wastes (ammonia) across thin permeable regions of the body wall. A variety of gas exchange structures, including the tube feet, is found in various echinoderms. A hemal system is present but its role in transport is still poorly understood and the chief transport system is the circulating fluid of the various coelomic compartments. The hemal system may be through transport system that delivers nutrients from the gut to these compartments for local distribution. The nervous system consists of two central intraepidermal nerve rings from which arise radial nerves to the periphery. Echinoderms are gonochoric and fertilization is usually external.
Crinoidea C
Crinoids are the stalked sea lilies and free-living feather stars. The taxon was abundant in Paleozoic seas but today crinoids are a declining group of only about 700 species.
Sea lilies (Fig 28-54A) account for the abundance of Paleozoic species and are the more typical crinoids even though even though only a few live today. Feather stars (Fig 28-55D), which are derived and all belong to Comatulida O, are the dominant crinoids in modern seas where they are represented by about 600 species.
The two groups (sea lilies and feather stars) share a common body plan except that sea lilies are attached to the bottom by a stalk whereas the feather stars have no stalk or other permanent attachment. Feather stars move freely over the substratum and even leave the bottom to swim. Sea lilies are found in deep water whereas feather stars are primarily shallow-water animals that are often be found in coastal areas.
Crinoids are radially symmetrical benthic suspension feeders. The tube feet of the branched arms are used to collect suspended material in the water column and transport it to the mouth. In life position crinoids are oriented upside down in comparison with other echinodermns, with their oral surface uppermost.
Laboratory Specimens
Almost any undergraduate invertebrate zoology that includes a crinoid laboratory exercise will employ feather stars rather than sea lilies. Neither is readily available but sea lilies are much less so than feather stars. Crinoids in general are most common in the Indo-Pacific and relatively few species occur in the Atlantic.
The comatulid Antedon bifida occurs along the European coast from the Mediterranean to Norway and Iceland. Several comatulidan species occur in the Caribbean and adjoining subtropical regions, including South Florida and the Bahamas. There are no crinoids on the Pacific coast of the United States but one species, in the genus Florametra, lives in British Columbia.
Antedon is typically used in laboratory studies of crinoids in Europe but there is no locally available crinoid species for most of the east coast of North America. Living comatulids occur in shallow water in the Caribbean and at least one, Comactinia echinoptera, lives in shallow water off the coast of south Florida. This account is written for Comactinia but parenthetical remarks are included for Antedon. Antedon and Comactinia are both feather stars in Comatulida but belong to different lower taxa (families and superfamilies). Antedon belongs to Antedonidae in Antedonacea. For our purposes the important differences lie primarily in the features of the oral disk.
The exercise can be conducted on preserved or living material. Living feather stars are difficult to relax and tend to curl into a ball when placed in magnesium chloride. Perform as much of the observation as possible using unrelaxed animals. If relaxation is necessary, add crystals of magnesium chloride to the seawater containing a living specimen and let it dissolve and slowly diffuse throughout the water.
Preserved material should be studied in a pan of tapwater.
External Anatomy
Make a preliminary examination of your specimen. The feather star body consists of a central globose crown from which radiate a set of 10 (usually) long, pinnately branched arms, and several short, unbranched cirri (Fig 1, 28-54B). The viscera, with the exception of the gonads, are located in the crown (Fig 28-58). The crown is divided into a hard, aboral calyx, to which the cirri and arms are attached, and a soft oral tegmen (Fig 28-54A). Both mouth and anus are on the oral side, on the tegmen.
Figure 1 The feather star, Comactinia echinoptera. Redrawn from Clark (1915). Crinoid7L.gif
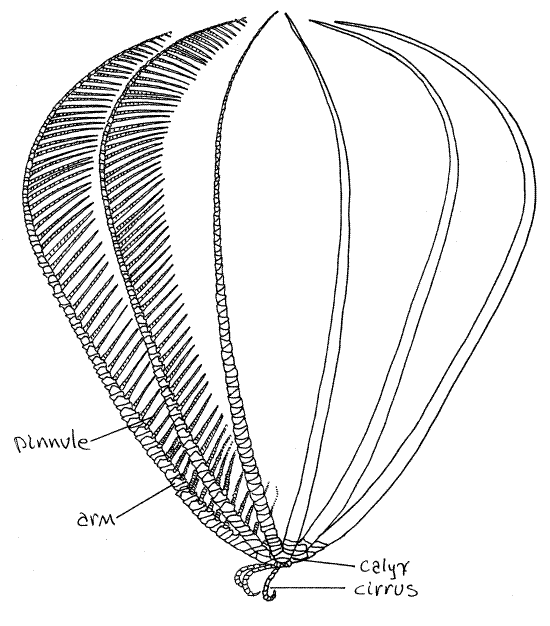
Crinoids are suspension feeders. In the feeding position (Fig 1) the cirri are used to hold the star to the rocky substratum and the oral surface faces up, away from the substratum. The arms are extended in the water. Ciliated ambulacral grooves on the oral surface of the arms lead to the mouth and are used to transport food collected by the arms.
Calyx
Use the dissecting microscope to look at the aboral surface. The calyx is covered by numerous calcareous calyx ossicles, or plates, and consequently is hard (Fig 28-59A). The plates are in the dermis, of course, and are part of the endoskeleton. Although it is not apparent, a thin cellular epidermis covers the outside of the plates.
Figure 2. Aboral view of the calyx of Comactinia echinoptera from St. Lucie Rocks, Florida. Crinoid4L.gif
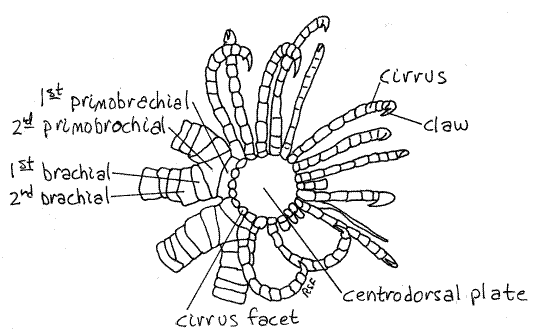
The most obvious of the calyx ossicles is the large disklike centrodorsal plate, which covers the center of the calyx and most of its periphery as well (Fig 2). This plate is, in reality, all that remains of the stalk of the sea lily ancestors of the comatulidans. As such it is the uppermost of a now lost column of similar calcareous disks that formed the stalk (Fig 28-54A). A stalk is present during the ontogeny of comatulidans but is lost, except for the centrodorsal plate, in adults.
Slender, jointed cirri radiate from the margin of the centrodorsal plate (Fig 1, 2, 28-59A). The cirri are used to hold the star in position on rocky substrata. They are composed of columns of calcareous ossicles arranged end to end like vertebrae in a vertebral column. The distal ossicle is a small claw.
Arms
The arms, or brachia, likewise are composed of columns of cylindrical vertebra-like ossicles laid end to end and covered with a thin epidermis and connected by connective tissue and muscles (Fig 1, 28-59B). The five arms arise in a whorl oral to the cirri and, in most crinoids, branch immediately into two to produce a total of 10 arms. In a few species they do not branch and in some others they branch repeatedly.
The unbranched bases of the arms can be seen emerging from the edges of the centrodorsal plate (Fig 2). The unbranched base consists of two ossicles, the first primobrachial and the second primobrachial (Fig 2) at the edge of the centrodorsal plate. The two branches arise from the second primodorsal plate. Each branch consists of a series of spool-like ossicles. The first of these is the first brachial, which articulates with the distal end of the second primobrachial.
The bases of the arms make up the sides of the crown whereas the centrodorsal plate is its aboral surface. The tegmen is the oral surface of the crown. The arms arise at the junction of the calyx and tegmen and the soft tissue of the tegmen can be seen insinuated between the arms and their branches.
Tegmen
Turn the star over to study the oral surface of the crown. It is covered by the soft tegmen (Fig 3). The central region of the tegmen is flat and bears the mouth and anus. This region is the disk. In Comasteridae, to which Comactinia (but not Antedon ) belongs, the anus is located in the center of the tegmen on a large anal papilla. The mouth is displaced to the side and is located at the edge of the disk. It is large but does not sit atop a papilla. (In Antedon the mouth is in the center of the disk and the anus is displaced to one side (Fig 28-55B).)
Figure 3. The tegmen of Comactinia. Crinoid5L.gif
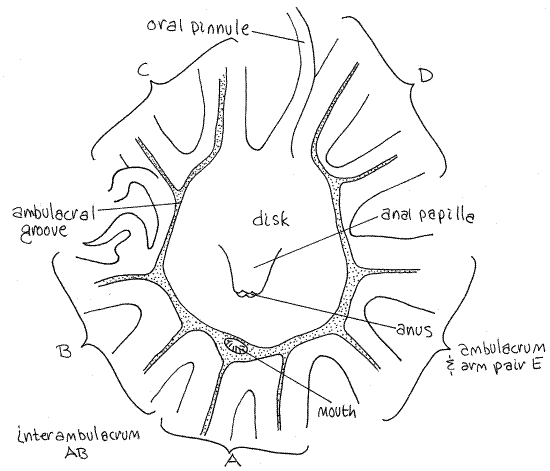
Crinoids do not have a conventional external madreporite. The tegmen surface is perforated by numerous tiny ciliated tegmenal pores which open into the perivisceral coelom (Fig 28-59A). Numerous small stone canals open from the water ring into the perivisceral coelom, which is connected to the outside via the tegmenal pores.
Symmetry
The presence of both mouth and anus on the oral surface disrupts the fundamental radial symmetry and the disk is bilaterally symmetrical. The plane of symmetry passes through the mouth and anus.
A plane passed through the mouth and anus will bisect (more or less) one arm and its ambulacrum (Fig 3). The bisected arm is the one adjacent to the mouth. On the side of the disk opposite the mouth the plane passes through an interambulacrum. The arm pair and ambulacrum next to the mouth are designated with an "A". The other arms are B, C, D, and E proceeding clockwise around the disk with the disk facing the observer (Fig 3). Thus the plane of symmetry passes through ambulacrum A and the interambulacrum between C and D.
Mouth and Ambulacra
In comatulaceans, such as Comactinia, the mouth is displaced to the edge of the disk and the ambulacra converge on it but they do so without crossing the disk. The ambulacra from the arms on the far side of the disk run around the margins of the disk to reach the mouth (Fig 3). Along the way they are joined by the ambulacral grooves from the other arms. The disk is thusalmost entirely encircled by the ambulacral groove but there is a small gap in the ring on the side directly opposite the mouth. The gap is in interambulacrum CD.
In the more primitive Antedon, a set of five ambulacral grooves radiates outward from the mouth at the center of the disk (Fig 28-55B). About halfway across the disk they bifurcate to provide each of the ten arms with its own ambulacrum in a radially symmetrical array.
Follow an ambulacral groove from the mouth out one of the arms. The margins of the ambulacra are shallowly scalloped. Each scallop is called a lappet and those on opposite sides alternate with each other (Fig 28-55C). The lappets can be extended to cover the ambulacrum.
The ambulacra bear small tube feet and are ciliated. Suspended food particles gathered from the water are transported by the cilia down the ambulacrum to the mouth.
Pinnules
Each arm bears numerous pinnately arranged branches called pinnules arrayed alternately along its sides (Fig 1, 58-55C,D). The ambulacra branch to extend along the oral midline of each arm and out onto most of the pinnules.
Three types of pinnules are present. Those closest to the disk are the proximal, or oral pinnules. They are larger than the others and are mechanoreceptive. They guard the disk and can arch over it and almost completely cover it. They do not have ambulacra or tube feet. A few pairs of oral pinnules are at the base of each arm.
Next, moving out the arm, are the genital pinnules. These contain the gonads and usually are thicker, especially near their bases, and especially when the gonads are ripe (Fig 28-55C). The genital pinnules occupy the middle region of the arm and have ambulacra and tube feet in Comactinia.
The distal pinnules occupy the outer end of the arm. They are numerous and usually thinner than the genital pinnules. They are the major feeding structures and always have ambulacra and tube feet (Fig 28-56A,B).
Internal Anatomy
Internal anatomy will not be considered in this exercise.
References
Bullough WS . 1958. Practical Invertebrate Anatomy, MacMillan, London. 483pp.
Chadwick HC. 1907. Antedon. Liverpool Mar. Biol. Comm. Mem. 15:1-47, pls. 1-7.
Clark AH. 1915. A monograph of existing crinoids 1.The comatulids, part 1. Bull. U.S. Nat. Mus. 82:1-406, pls.1-17.
Clark AH. 1921. A monograph of existing crinoids 1.The comatulids, part 2. Bull. U.S. Nat. Mus. 82:1-795, pls 1-57.
Heinzeller T, Welsch U. 1994. Crinoidea, pp. 9-148 in Harrison FW, Chia FS (eds.). Microscopic Anatomy of Invertebrates vol. 14 Echinodermata. Wiley-Liss, New York. 510p.
Hyman LH . 1955. The Invertebrates IV. Echinodermata . McGraw-Hill, New York. 763pp.
Ruppert EE, Fox RS, Barnes RB. 2004. Invertebrate Zoology, A functional evolutionary approach, 7 th ed. Brooks Cole Thomson, Belmont CA. 963 pp.
Supplies
Dissecting microscope
Living or preserved crinoid
Magnesium chloride crystals for living specimens