Cerebratulus ©
Ribbonworm
Invertebrate Anatomy OnLine
Cerebratulus ©
Ribbonworm
30jun2006
Copyright 2001 by
Richard Fox
Lander University
Preface
This is one of many exercises available from Invertebrate Anatomy OnLine , an Internet laboratory manual for courses in Invertebrate Zoology. Additional exercises can be accessed by clicking on the links on the left. A glossary and chapters on supplies and laboratory techniques are also available. Terminology and phylogeny used in these exercises correspond to usage in the Invertebrate Zoology textbook by Ruppert, Fox, and Barnes (2004). Hyphenated figure callouts refer to figures in the textbook. Callouts that are not hyphenated refer to figures embedded in the exercise. The glossary includes terms from this textbook as well as the laboratory exercises.
Systematics
Nemertea P, Anopla C, Hetronemertea O, Lineidae F (Fig 11-13)
Nemertea P
Nemertean worms are active, benthic predators that use an eversible, sticky or barbed, and sometimes poisonous proboscis to capture prey (Fig 11-4). Nemerteans are long and slender, aptly known as ribbon worms, or rubber band worms. The longest is about 50 m but most are much less than that, usually no more than 20 cm. Many are brightly colored. Most of the 1150 species are marine but a few live in freshwater or terrestrial habitats.
The gut is complete and extends from anterior mouth to posterior anus. The long proboscis is housed in a cavity, the rhynchocoel, from which it is everted by hydrostatic pressure generated by surrounding muscles. The tubular proboscis everts, by turning inside out, from an anterior proboscis pore which may or may not be independent of the mouth.
An anterior brain and a pair of longitudinal lateral nerve cords are present. Excretion is via protonephridia. Nemerteans are gonochoric. The body wall is complex with numerous layers of muscles, epithelia, spaces, and connective tissue, and varies with order. There is no cuticle.
Nemerteans have traditionally been thought of as having a compact body plan because the viscera do not appear to be enclosed in a body cavity. Recent studies however have revealed the presence of a coelom and necessitate the reevaluation of the phylogenetic affinities of these animals. The fluid transport system has been shown to be a coelomic space. The rhynchocoel is also a coelomic space.
Anopla C
These are the unarmed nemerteans whose proboscis lacks stylets. The mouth is posterior to the brain. Anopla is a paraphyletic taxon.
Heteronemertea O
The heteronemertean body wall comprises four muscles layers with the lateral nerve cords located in the innermost circular layer.
Laboratory Specimens
Cerebratulus can be large enough to be suitable for dissection in teaching laboratories although it is rarely available in numbers sufficient to support such use. It can be collected in coastal sediments or purchased living or preserved from biological supply companies.
Cerebratulus is represented by over 100 species, many of which are very large, some reaching many meters. On the east coast of North America the only large species is Cerebratulus lacteus but the west coast has several large species suitable for dissection. The dissection is best performed using a living specimen but preserved material will serve if necessary.
Behavior
Living specimens should be observed in aquaria or dishes of seawater before being anesthetized. Specimens should be handled carefully as many large species tend to break into fragments or evert their proboscis when handled. Should the proboscis be everted during the exercise, note the extreme stickiness of its mucus. It is nearly impossible for you to free it from a glass surface, for example, and very difficult to detach from your finger should it be touched. Touch it! The worm, however, can release its sticky hold at will and retract the proboscis back into the body.
One of the distinguishing characteristics of Cerebratulus is its ability to swim propelled by undulations of the body. To facilitate swimming the body is flattened dorsoventrally and has wide, flat, lateral margins. Watch a Cerebratulus swim in an aquarium.
Normally Cerebratulus lives in soft sediments where it burrows using retrograde peristaltic contractions of the body wall muscles. Place a specimen in a large dish of fine sand and seawater and watch it burrow.
Anatomy
Carefully place a specimen in a 4" culture dish of clean seawater and take it to your desk. Add a gram or two of magnesium chloride crystals to the center of the dish and let the motion of the worm gradually mix the chemical with the water. Continue observing the worm and its movements.
Watch for retrograde (anterior to posterior) peristaltic contractions as the animal responds to the relaxant. Normally these waves are used for burrowing (rapidly). Watch also for production of a copious mucus. Mucus from the body surface is much less sticky than that from the proboscis. Set the dish aside and do something else until the worm is relaxed.
When your specimen is completely relaxed and no longer responds to stimuli (1-2 hours), place the dish on the stage of the dissecting microscope.
External Anatomy
Cerebratulus is long and dorsoventrally flattened. The body surface is ciliated. The weakly developed head is at the anterior end. The posterior end is often missing but if your worm is complete, there will be a short, filamentous, tail-like caudal cirrus extending from the posterior end of the body. The body is wide and has thin flattened lateral margins to facilitate swimming.
The extreme anterior end of the body is the head. It is poorly developed but may be set off from the rest of the body by a slight constriction (Fig 1). The lateral margins of the head are deeply split by two ciliated chemosensory lateral cephalic slits, one on each side (Fig 1, 11-9A). The ciliary current in the slits runs from anterior to posterior. It enters at the anterior tip of the head and exits at the posterior end of the slit.
Figure 1. Ventral view of the anterior end of the heteronemertean Cerebratulus from Coos Bay, Oregon. Nemertea7La.gif
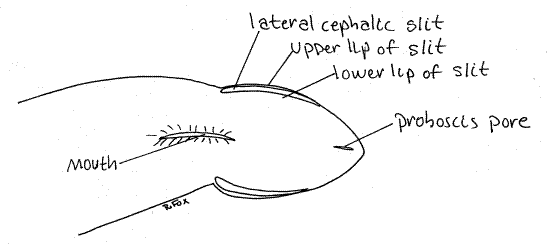
Under magnification place a drop of isotonic seawater/milk suspension to the water in front of the head and watch for its slow entry into the cephalic slit. Watch the posterior end of the slit for its reappearance.
Look at the ventral surface of the head. The tiny proboscis pore is located at the anterior end on the ventral surface (Fig 1, 11-2A,B). It is a midventral, longitudinal slit that is usually closed. Open it with minuten nadel and fine forceps to reveal the proboscis lumen. The proboscis is everted through the proboscis pore.
The proboscis pore and mouth open independently of each other in Cerebratulus and other Anopla. The mouth, also ventral, is much larger than the proboscis port (Fig 1). It is farther posterior, located at about the level of the posterior end of the lateral cephalic grooves. It is also a median, longitudinal slit. It opens into the foregut. In Anopla the mouth is posterior to the brain.
The remainder of the body is undifferentiated and nearly featureless although the anterior end is rounder in cross section and the posterior end flatter. The anus and short, threadlikecaudal cirrus are at the posterior end. You will not see either if the posterior end of the worm is missing, as is often the case.
Internal Anatomy
Place the worm dorsal side down in a long, narrow, wax-bottom dissecting pan. Use two #1 insect pins placed through the edges of the body beside the mouth to hold the worm in place. The head should be at one end of the pan.
Digestive System
The first region of the gut is the ectodermal pharynx (Fig 11-5A). It is ridged longitudinally. Open the mouth with fine forceps and nadel and look inside to see the cavity and its ridges.
" With the worm on the stage of the dissecting microscope, insert one tip of a fine scissors in the mouth and cut posteriorly along the ventral midline, through the body wall, thus opening the gut lumen. Pin the walls of the gut (and body wall) aside as you go. Use # 1 stainless steel insect pins and insert them into the wax at 45° angles. Find the pharynx again (Fig 11-5A). Note that the diameter of this region is enormous, filling most of the interior of the worm. It is so large that you may get the impression you are opening a coelomic cavity but you are not. Notice the conspicuous large longitudinal ridge running like a typhlosole along the dorsal midline of the gut. This is the proboscis and proboscis sheath bulging into the gut lumen (Fig 11-2B).
Moving posteriorly the pharynx soon becomes the stomach but the two are similar and difficult to differentiate. Both are derived from ectoderm and are part of the foregut. The pharynx is more heavily ridged than the stomach, the walls of the stomach being relatively smooth.
Continue cutting posteriorly until the spacious stomach ends abruptly with a transverse bulkhead, or sphincter, partially occluding its lumen. Posterior to the stomach is the endodermalintestine, or midgut, whose apparent diameter is less than that of the stomach. Its walls are folded to form a series of pouchlike diverticula extending to the body wall. The intestine is characterized by the presence of these diverticula. In living specimens the epithelium of the intestine may be white, whereas that of the stomach is often pinkish.
Continue cutting posteriorly, noting that the dorsal ridge representing the proboscis is still present. The intestine continues for most of the remainder of the length of the body. The dorsal bulge becomes very large in the middle region of the intestine. As you approach the posterior end of the body you should notice changes in the character of the ridge (proboscis). It becomes smaller and flatter and its color changes.
Near the posterior end of the worm, the intestine opens into the short, ectodermal rectum. The rectum has no diverticula. It opens to the exterior via the anus at the posterior end of the body.
Reproductive System
Cerebratulus, like most nemerteans, is gonochoric. Its numerous paired gonads are located in pouches in the body wall between successive intestinal diverticula (Fig 11-2A). During periods of reproductive activity, a temporary gonoduct and gonopore develop for each gonad. Through them gametes are shed to the sea and fertilization is external. Are gonads present over the entire length of the worm or are they restricted to certain regions?
Mentally compare the simplicity of the nemertean reproductive system with the amazing complexity (and diversity) of that of platyhelminth worms (Fig 10-24, 10-37).
If gametes are present in the gonads, make a wet mount with seawater and examine them with the dissecting microscope. Look for gametes and determine if your specimen has eggs or sperm. Eggs are large spheres with visible nuclei. Sperms are tiny flagellated cells. Is your specimen a male or female?
Rhynchocoel and Proboscis
Turn to the anterior end of the worm. Find the anterior end of the typhlosole, or ridge, in the roof of the pharynx and open it with a longitudinal cut made with your fine scissors. This ridge, remember, contains the rhynchocoel and proboscis. Its wall, through which you just cut, is the proboscis sheath (Fig 11-2A, B). The cavity exposed by the cut is the rhynchocoel. Inside it you can see the large, very long proboscis. (If the rhynchocoel is empty, it means the worm has everted and then lost the proboscis. This sometimes happens during collection and handling of specimens.) The rhynchocoel is a coelomic space.
Continue cutting posteriorly, opening the rhynchocoel and exposing the proboscis for the entire length of the gut. Note that in the areas where the typhlosole is very thick the proboscis is coiled or convoluted. Near the posterior end, the proboscis diminishes in diameter and ultimately attaches to the walls of the rhynchocoel. There is no separate proboscis retractor muscle inCerebratulus, instead the muscular posterior end of the proboscis itself acts as the retractor.
Back at the anterior end of the worm, extend the longitudinal incision to the proboscis pore. In doing this you will cut through nerve ring and expose the lateral lobes of the brain. In living specimens the brain is red with neuroglobin but in preserved material it is white. Neuroglobin is a type of non-circulating hemoglobin that stores oxygen for use by the brain.
Having opened the rhynchocoel anteriorly to the proboscis pore you can now see that the proboscis pore opens into a cavity, the rhynchodeum, occupying most of the interior of the head (Fig 11-2A, B). The rhynchodeum narrows posteriorly and is continuous with the lumen of the proboscis. Where it narrows, the walls of the proboscis join with the body wall. This is the anterior point of attachment of the proboscis to the body. The proboscis is, in fact, a deep invagination of the body wall and its walls are composed of the same muscle and epithelia layers as the body wall. The retractor muscle or, in the case of Cerebratulus the posterior end of the proboscis, attaches posteriorly to the proboscis sheath.
Carefully remove the proboscis from the rhynchocoel, extend it and compare its total length with that of the worm.
Nervous System
The nervous system consists of a large dorsolateral brain and two lateral longitudinal nerve cords (Fig 11-2A). You may have seen the brain already. In life it is red with neuroglobin but in preserved material is white. In Cerebratulus the brain is located immediately anterior to the mouth and almost exactly level with the anterior point of attachment of the proboscis with the proboscis sheath. The two large lateral ganglia are joined to each other by a large dorsal and small ventral commissures to form a nerve ring around the proboscis (Fig 11-2B). Note that the nerve ring is around the proboscis, rather than the gut.
A large lateral nerve cord exits each of the lateral ganglia and extends posteriorly for the length of the worm. The nerve cords are easily accessible by dissection from the gut lumen. You can find them by cutting or tearing through the lateral wall of the buccal cavity.
Fluid Transport System
There is a well-developed fluid transport system with narrow, well-defined coelomic vessels connecting large sinuses, or lacunae (Fig 11-7C). There is a large cephalic lacuna in the head. Two lateral vessels run posteriorly from the cephalic lacuna. There is also a median, dorsal, longitudinal vessel. It has been shown that the vessels are coelomic channels rather than blood vessels. The channels are lined by a ciliated mesothelium. The vessels will not be apparent in your dissection.
Excretory System
The excretory system consists of protonephridia with flame bulbs associated with the lateral coelomic vessels (Fig 11-8). They will not be seen in this dissection.
References
Goodchild CG. 1950. Amphiporus ochraceous, pp 209-214 in Brown FA (ed). Selected Invertebrate Types. Wiley, New York. 597p.
Coe WR. 1895. Anatomy of a species of nemertean (Cerebratulus lacteus Verrill) with remarks on certain other species. Trans. Connecticut Acad. Sci. 9:479-514, pls. 10-15.
Coe WR. 1905. Nemerteans of the west and northwest coasts of America. Bull. Mus. Comp. Zool, Harvard 47:1-320, pls. 1-25.
Coe WR. 1937. Methods for the laboratory culture of Nemertea. pp.162-165 in Needham JG. et al. Culture Methods for Invertebrate Animals. Comstock, Ithaca.
Coe WR. 1943. Biology of the nemerteans of the west coast of North America. Trans. Conn. Acad. Arts Sci. 35:129-328, pls. 1-4.
Pennak RW . 1989. Fresh-water Invertebrates of the United States, 3 rd ed. Wiley, New York. 628p.
Ruppert EE, Fox RS. 1988. Seashore animals of the southeast. Univ. South Carolina Press, Columbia, 429.
Ruppert EE, Fox RS, Barnes RB. 2004. Invertebrate Zoology, A functional evolutionary approach, 7 th ed. Brooks Cole Thomson, Belmont CA. 963 pp.
Turbeville JM. 1991. Nemertinea, pp. 285-328 in Harrison FW, Bogitsh BJ (eds.). Microscopic Anatomy of Invertebrates vol. 3 Platyhelminthes and Nemertinea . Wiley-Liss, New York.
Turbeville JM, Ruppert EE . 1985. Comparative ultrastructure and the evolution of nemertines. American Zool. 25-71.
Supplies
Dissecting microscope
Large living or preserved Cerebratulus
Seawater for living specimens
Magnesium chloride crystals if using living specimens
Wax-bottom dissecting pan made from kippered herring tins or aluminum ice trays
Milk made isotonic to seawater by adding salts
Plastic Pasteur pipet
Dissecting set with microdissecting tools
Stainless steel # 1 insect pins