Invertebrate Anatomy OnLine
Ceratomia catalpae ©
Catalpa Caterpillar
30jun2006
Copyright 2001 by
Richard Fox
Lander University
Preface
This is one of many exercises available from Invertebrate Anatomy OnLine , an Internet laboratory manual for courses in Invertebrate Zoology. Additional exercises can be accessed by clicking on the links in the column on the left. A glossary and chapters on supplies and laboratory techniques are also available. Terminology and phylogeny used in these exercises correspond to usage in the Invertebrate Zoology textbook by Ruppert, Fox, and Barnes (2004). Hyphenated figure callouts refer to figures in the textbook. Callouts that are not hyphenated refer to figures embedded in the exercise. The glossary includes terms from this textbook as well as the laboratory exercises.
Systematics
Arthropoda P, Mandibulata, Tracheata, Hexapoda SC, Insecta C, Dicondylia, Pterygota, Metapterygota, Neoptera, Eumetabola, Holometabola, Lepidoptera O, Sphingoidea SF, Sphingidae F, Sphinginae sF (Fig 16-15, 20-14, 20-15, 21-23)
Arthropoda
Arthropoda, by far the largest and most diverse animal taxon, includes chelicerates, insects, myriapods, and crustaceans as well as many extinct taxa. The body is segmented and primitively bears a pair of jointed appendages on each segment. The epidermis secretes a complex cuticular exoskeleton which must be molted to permit increase in size. Extant arthropods exhibit regional specialization in the structure and function of segments and appendages. The body is typically divided into a head and trunk, of which the trunk is often itself divided into thorax and abdomen.
The gut consists of foregut, midgut, and hindgut and extends the length of the body from anterior mouth to posterior anus. Foregut and hindgut are epidermal invaginations, being derived from the embryonic stomodeum and proctodeum respectively, and are lined by cuticle, as are all epidermal surfaces. The midgut is endodermal and is responsible for most enzyme secretion, hydrolysis, and absorption.
The coelom is reduced to small spaces associated with the gonads and kidney. The functional body cavity is a spacious hemocoel divided by a horizontal diaphragm into a dorsal pericardial sinus and a much larger perivisceral sinus. Sometimes there is a small ventral perineural sinus surrounding the ventral nerve cord.
The hemal system includes a dorsal, contractile, tubular, ostiate heart that pumps blood to and from the hemocoel. Excretory organs vary with taxon and include Malpighian tubules, saccate nephridia, and nephrocytes. Respiratory organs also vary with taxon and include many types of gills, book lungs, and tracheae.
The nervous system consists of a dorsal, anterior brain of two or three pairs of ganglia, circumenteric connectives, and a paired ventral nerve cord with segmental ganglia and segmental peripheral nerves. Various degrees of condensation and cephalization are found in different taxa.
Development is derived with centrolecithal eggs and superficial cleavage. There is frequently a larva although development is direct in many. Juveniles pass through a series of instars separated by molts until reaching the adult size and reproductive condition. At this time molting and growth may cease or continue, depending on taxon.
Mandibulata
Mandibulata includes arthropods in which the third head segment bears a pair of mandibles. As currently conceived this taxon includes myriapods, hexapods, and crustaceans. Appendages may be uni- or biramous and habitats include marine, freshwater, terrestrial, and aerial.
Tracheata
Myriapods and hexapods share tracheae and a single pair of antennae and are sister taxa in Tracheata. Crustaceans, which have gills and lack tracheae, are excluded and form the sister group.
Hexapoda
The body is divided into three tagmata; head, thorax, and abdomen (Fig 21-1). Appendages are uniramous and a single pair of antennae is present. Three pairs of legs and two pairs of wings are found on the thorax of most adults. Hexapod legs are uniramous although there is increasing evidence that they evolved from multiramous appendages of their ancestors. Gas exchange is accomplished by trachea. Excretory organs are Malpighian tubules and the end product of nitrogen metabolism is uric acid. There is relatively little cephalization of the nervous system. Insects are gonochoric with copulation and internal fertilization.
Insecta
Most hexapods are insects. A few hexapod taxa (orders) lack wings and have primitive mouthparts recessed into the head and belong to Entognatha, the sister taxon of Insecta. Insects have ectognath mouthparts and the adults (imagoes) of most taxa have wings.
Pterygota
The winged insects. These insects are derived from a winged common ancestor. Adults of most taxa have wings although they have been lost in some.
Eumetabola
Juveniles have no ocelli and there are six or fewer Malpighian tubules.
Holometabola
The final larval instar pupates and undergoes a radical metamorphosis in which it is converted to an imago, or adult. The imago is sexually mature and in most taxa has wings whereas larvae are immature and wingless. During metamorphosis many or most larval tissues are dismantled and adult structures built anew. Wings, for example, are manufactured from clusters of undifferentiated cells known as imaginal discs but not from preformed wingpads as in pauro- and hemimetabolous insects.
Lepidoptera O
Butterflies and moths. Order consisting of the polyphyletic “moths”, skippers (Hesperoidea), and scudders (Papilionoidea). Skippers and scudders are together known as “ butterflies”. Wings, body, appendages covered with pigmented, dust-like epidermal scales or hair-like setae. Adults with highly derived sucking mouthparts and liquid diet. Adult mouthparts consist of large labial palps and a coiled tubular proboscis derived from the maxillary galeae. Mandibles absent. Larvae herbivorous with typical chewing mouthparts. Larval labial glands modified as silk glands. Holometabolous complete metamorphosis in a pupal stage. In many moths pupation occurs in a silk cocoon but most butterflies have no cocoon and the pupa is known as a chrysalis, sometimes associated with a few silk fibers. Lepidopteran pupae are obtect, with the appendages attached to the body over their entire length.
Introduction
The Catalpa Caterpillar, or Catalpa "worm", and its adult, the Catalpa Sphinx, can be locally abundant during the summer months in the eastern United States and as far west as Colorado, Kansas, Nebraska, and Texas, wherever its two host trees, Catalpa bignoniodes (the Southern Catalpa) and C. speciosa (the Northern Catalpa), are found. These caterpillars are well known to anglers, who esteem them for fish bait (catfish, sunfish), and to homeowners, who lament the damage inflicted to catalpas, which can be completely defoliated.
Reaching a maximum length of 75 mm, late instar caterpillars are large enough to be easily dissected with the aid of a dissecting microscope. This caterpillar, a hornworm, is the larva of a sphinx, or hawk, moth in the family Sphingidae known as the Catalpa Sphinx. Hornworms have a prominent posterior dorsal tail spine, or horn, on the eighth abdominal segment (Fig. 1). The spine is not a stinger and the caterpillars do not sting.
The body is smooth with inconspicuous setae and late instar caterpillars occur in two color phases. The dark phase has a black head capsule and a wide black dorsal longitudinal stripe. The tail spine is black. The sides are chartreuse and the venter is pale green. The pale phase lacks the dorsal black stripe although it may have some dorsal black markings. Early instars are pale with black markings and a black tail spine. The final instar larvae leave their natal tree and pupate in the ground. There are five larval instars. Metamorphosis takes about 2 weeks in the summer but fall pupae overwinter and emerge in the spring. In the Deep South there may be as many as four generations (flights) each year. Photographs and discussion are available at Hyche (1994) and Oehlke (undated).
Catalpa worms are attacked by the braconid wasp, Apanteles congregatus (= Cotesia congregata), a parasitoid whose larvae live in the hemocoel of the caterpillar. A caterpillar 55 mm in length dissected during the preparation of this exercise contained 126 wasp larvae, presumably Apanteles, and a single, somewhat larger, fly larva in the hemocoel. Apanteles preys on at least 14 species of sphingid caterpillars, including the tomato and tobacco hornworms, Manduca spp,
Leaves damaged by catalpa worms secrete sugar from extrafloral nectaries. This attracts parasitoid wasps and ants, which attack the caterpillars (Ness, 2002). It also attracts honeybees.
The moth family Sphingidae includes about 1100 species of insects known generally as hawkmoths, sphinx moths, or hummingbird moths as adults and hornworms as caterpillars. The family includes the Tomato and Tobacco Hornworms and the Catalpa Caterpillar.
Like that of most moths, the life cycle consists of egg, five larval instars, pupa, and imago. Moths are holometabolous insects in which the body undergoes a radical metamorphosis during the pupal instar (Fig 21-14*). During metamorphosis wings develop de novo from imaginal discs (clusters of undifferentiated embryonic cells) in the larval thorax and muscles are reorganized for flight. The mouthparts are converted from the primitive chewing design suited for feeding on plant tissue to highly derived sucking mouthparts for ingesting liquids. The gut is reorganized to accommodate differences in larval and adult diets. The antennae form anew from imaginal discs in the head and the reproductive system develops. The prolegs are completely lost and the adult compound eye develops near the old larval ommatidia. The nervous system is reorganized, in part through condensation and cephalization of ganglia.
This exercise is written for living or freshly sacrificed late instar larvae but preserved caterpillars can be used. Color and texture descriptions may not be applicable to preserved material. The dissection should be conducted with a dissecting microscope using recently collected larva with food in its gut.
External Anatomy
Examine a living, active (i.e. unanesthetized) late instar larva of the catalpa sphinx, Ceratomia catalpae. Active caterpillars are difficult to manipulate but, with a little patience, most aspects of the external anatomy can be studied without anesthetization and it is best to do so to take advantage of the opportunity to observe locomotion and behavior. (If desired, however, the caterpillar may be anesthetized immediately to avoid the inconvenience of dealing with an uncooperative specimen.) With a little patience, the external anatomy can be studied without anesthetizing the specimen. Sometimes the caterpillar will sit quietly on the tip of your index finger, which can then be orientated under the lens of a dissecting microscope to provide the desired view of the specimen.
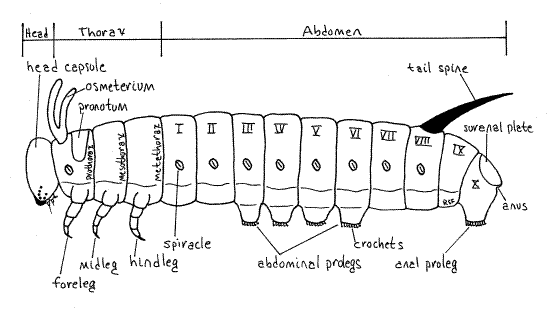
As expected, the larva is caterpillar-shaped, i.e. eruciform, in having a more or less cylindrical body and well-developed sclerotized head capsule, thoracic legs and abdominal prolegs (Fig. 1). The body is smooth and naked, with inconspicuous, scattered, short setae visible only with magnification. The body consists of a small anterior head, middle thorax, and large posterior abdomen (Fig 16-2*). The thorax and abdomen together make up the trunk. The head is connected to the thorax by a short, soft neck, or cervix. Paired, jointed, segmental appendages are present on the head and thorax but are lacking on the caterpillar abdomen. Paired, unjointed, segmental, fleshy prolegs are found on some abdominal segments. The body wall is heavily pigmented and opaque so that dissection is necessary to study the internal anatomy.
Figure 1. External anatomy of a generalized caterpillar. Redrawn and modified from Snodgrass (1935). Lepid63L.gif
Head
The head is enclosed in a black sclerotized head capsule, or epicranium, at the anterior end (Fig. 1, 21-1A,B). It consists of several fused segments and bears the appendages of those segments. The component segments of the head are not recognizable and the head appears to be unsegmented.
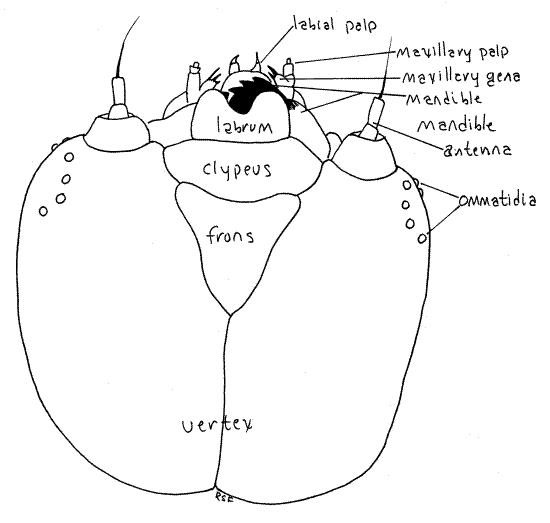
The posterior dorsum of the epicranium is the vertex, the sides are the genae, and the front is the frons (Fig. 2). A conspicuous Y-shaped epicranial suture divides the capsule into right and left halves. The fork of the “ Y” is formed by the two frontal sutures whereas the upright of the “Y” is the coronal suture. The triangular frons and the clypeus lie between the two frontal sutures on the front of the head capsule. The clypeus is situated on the ventral border of the frons. The bilobed labrum articulates with the ventral edge of the clypeus. The labrum forms the anterior border of the preoral cavity, which is a space surrounding the mouth and formed by the ring of mouthparts around the mouth: labrum anteriorly, mandibles and maxillae laterally, and labium posteriorly (Fig 21-7).
Insects are usually considered to have three pairs of segmental appendages that function as mouthparts; mandibles, maxillae, and labium. Most entomologists do not consider the labrum to be a segmental appendage although some do. Posterior and ventral to the labrum are the large, dark, very heavily sclerotized mandibles, each with a set of powerful median teeth. The teeth of the two mandibles oppose each other on the midline and are used to cut tissue from the catalpa leaf. If your specimen is alive and active you may see the mandibles move in the transverse plane. The mandibles form the sides of the preoral cavity and lie beside the mouth. Use a pair of fine forceps to move the mandibles apart and reveal the mouth. The mouth is plugged by the soft hypopharynx, or tongue immediately posterior to it (Fig 21-7). With the mandibles held aside push the hypopharynx posteriorly to open the mouth. The hypopharynx is a fold of the body wall and is not a segmental appendage and is not paired. The mandibles, mouth, and hypopharynx will be easier to study after you have anesthetized your specimen.
Orient the caterpillar on its back with its venter facing up, toward you, and study the two remaining mouthparts. The maxillae and labium are small, inconspicuous, and best studied after the specimen is anesthetized. Immediately posterior to each mandible is a maxilla consisting of a sclerotized basal stipes from which protrudes a tiny, lateral, biarticulate maxillary palp and a medial galea bearing strong apical spines. During metamorphosis the galea is transformed into the large sucking proboscis characteristic of adult lepidopterans. The labium, which is derived from a fused pair of posterior head appendages, lies on the midline between the two maxillae. Its large bulbous anterior portion bears a pair of vestigial labial palps at its base. These palps become greatly enlarged in adults and, along with the maxillary proboscis, are the only functional adult mouthparts. The median, inconspicuous spinneret lies between the two palps.
Figure 2. Dorsal view of the head capsule of the Catalpa Caterpillar, Ceratomia catalpae. Lepid57L.gif
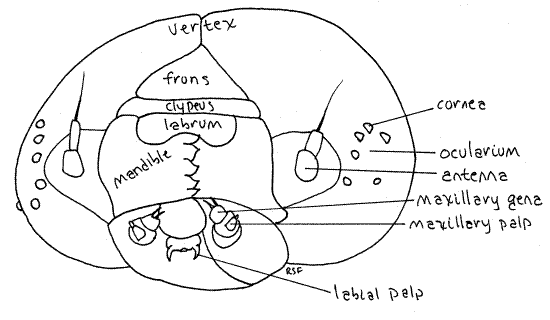
Antero-laterally each side of the capsule bears an ocularium consisting of a C-shaped ring of the colorless, transparent corneae, or lenses, of six large isolated ommatidia, sometimes erroneously referred to as ocelli but there are no true ocelli in lepidopterans (Fig. 3). The ocularium is a derived compound eye with six discontiguous facets. The ommatidia are best found by using higher magnification to look at the anterior corner of the sides of the head capsule.
Posteriorly the head capsule is penetrated by a large opening, the foramen magnum, through which the gut, nerve cord, silk ducts, hemocoel and tracheae pass between head and thorax. The foramen is filled with he soft tissue of the cervix and is obscured when the head is attached to the thorax but you can make out it's outline by pushing the soft tissue of the cervix aside. With fine forceps, pull the head forward to expose the soft, unsclerotized cervix connecting it with the trunk.
A short, inconspicuous, cylindrical antenna can be seen lateral to each mandible. The antennae are between the mandibles and the ommatidia. The antennae and ommatidia should be revisited after you have anesthetized the specimen and it ceases to move.
Thorax
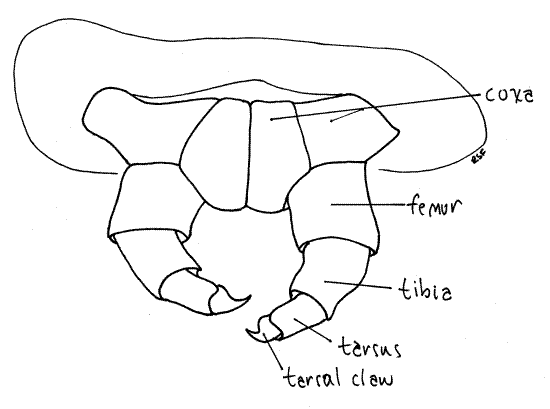
The insect thorax consists of three segments, prothorax, mesothorax, and metathorax, each of which bears a pair of sclerotized, jointed thoracic legs known as the forelegs,midlegs, and hindlegs, respectively (Fig. 1). In caterpillars, each thoracic leg consists of a series of cuticular cylinders decreasing in diameter distally (Fig. 5, 21-1E). The largest ring is the proximal coxa, which articulates with the body (Fig. 4). It is followed in turn by the femur, which is fused with a vestigial trochanter, then the tibia, and finally a biarticulate tarsus. The terminal tarsal article is a darkened, sclerotized, hooked claw.
Figure 3. En face view of the head of Ceratomia catalpae. Dorsal is up. Lepid58L.gif
The prothorax bears a cuticularized pronotum, or prothoracic shield, covering its dorsal surface. An oval white prothoracic spiracle can be seen on each side near the edge of the shield (Fig. 1). These are actually the spiracles of the mesothoracic segment that have migrated anteriorly. The mesothorax and metathorax have no sclerotized nota nor spiracles.
Figure 4. The forelegs of Ceratomia catalpae. Lepid59L.gif
Abdomen
The abdomen consists of a succession of ten segments (Fig. 1). Most of the abdomen is loaf-shaped (rounded on top and flattened ventrally) in cross section but posteriorly it is slightly flattened. Abdominal segments 3-6 and 10 each bear a pair of soft abdominal prolegs. Those of the 10 th segment lie beside the anus and are known as anal prolegs. The prolegs make good landmarks for recognizing the segments by number. Each proleg terminates in a central sucker surrounded by two parallel curved rows of sclerotized hooks, or crochets. The crochets of the lateral row are much heavier and darker than those of the median row. The suckers can be used to grasp smooth surfaces whereas the crochets function primarily in attaching to silk or other rough surfaces. Under the influence of normal blood pressures the crochets engage the substratum and attach. Elevated blood pressure causes the crochets to disengage. Consequently, at rest the larva is attached to is substratum and unlikely to fall. An active elevation of hemocoelic pressure is required to disengage.
Abdominal segments 1-8 each bear a pair of oval white spiracles which open into the extensive tracheal system (Fig. 1). Segment eight bears the prominent, eponymous horn, or tail spine, dorsally.
Segment nine lacks either spiracles or prolegs. Segment 10 is dorso-ventrally flattened, bears a pair of anal prolegs and is covered by a dorsal, triangular, sclerotized, but not darkened, suranal sclerite, or suranal plate (Fig. 1, 5). Find the anus hidden beneath the suranal sclerite.
If it is alive, your caterpillar may release one or more fecal pellets. If so, examine one at about 20X and demonstrate to yourself that it is enclosed in a delicate peritrophic membrane. Inside the membrane you will find the uniformly sized and shaped bites of plant tissue cut from the host plant by the mandibles.
Internal Anatomy
The remainder of the exercise should be performed on an anesthetized or recently sacrificed specimen. It makes little difference which. Anesthetize (or kill) a caterpillar by placing it in 7% non-denatured ethanol in a small wax-bottom dissecting pan. Empty anchovy fillet tins poured with a wax bottom make ideal dissecting pans for most insects. Spend a few minutes observing features that were difficult to see, such as the ommatidia, head appendages, mouth, spiracles, thoracic legs, prolegs, crochets, and anus earlier while the animal was active.
If your specimen shows any signs of life it may periodically extend and retract the crochets of the prolegs. Watch a circle of crochets under high magnification of a dissecting microscope during one of these cycles. At rest the curved crochets are deployed and would engage silk strands or another rough substratum. When withdrawn they release their hold on the substratum and permit movement of the proleg.
Examine a spiracle at about 40X. Each spiracle consists of a brownish, sclerotized, cuticular ring surrounding a recessed atrium from which tracheae arises The atrium is covered and protected by a filter apparatus consisting of a ring of closely spaced bristles. The atrium is under the filter and cannot be seen from the surface. A slit-like atrial orifice penetrates the center of the filter and opens into the atrium below it. Insert a minuten nadel into the orifice to demonstrate its presence and continuity with the atrium. Use the nadel to demonstrate that the filter is composed of bristles extending toward the orifice from the surrounding ring. In most insects, including Ceratomia, the spiracle is equipped with a valve, under muscular control, that is important in water conservation as it allows the animal to control evaporation from the tracheal system.
Find the articles (coxa, femur, tibia, tarsus with terminal claw) of a thoracic leg using 40X (Fig 4).
The insect body cavity is the hemocoel (Fig 16-7), which is filled with circulating blood that bathes the tissues and transports food, wastes, and hormones (but not oxygen). Organs and tissues, including the gut, nerve cord, fat bodies, salivary glands, and excretory organs, are suspended in the hemocoel and surrounded by blood. The hemocoel is divided by two horizontal septa (dorsal and ventral diaphragms) into three sinuses. The dorsal pericardial sinus encloses the heart, the middle perivisceral sinus is the largest and contains the gut and other viscera. The small, ventral perineural sinus surrounds the ventral nerve cord. The following dissection opens the hemocoel for a study of its organs.
" To begin the dissection, insert one blade of a fine scissors under the posterior edge of the suranal sclerite and cut anteriorly through the body wall well to the right of the middorsal line so that the heart and pericardial sinus are not damaged. Make this cut along the right edge of the dorsal black stripe. Use #1 stainless steel insect pins to fasten the cut edges of the body wall to the floor of the dissecting pan. Begin at the posterior end and work your way anteriorly, inserting each pin at a 45 ° angle and stretching the body wall laterally and posteriorly as you pin it.
The heart will end up on the left side of the incision. Remember this so you can find it later after it is pinned to the bottom of the dissecting pan. The greenish-brown perivisceral sinus and dorsal blood vessel will ideally remain attached to the dorsal body wall but what is more likely is that they will adhere to the mass of fat body and connective tissue surrounding the gut and be torn away from the body wall. Watch for these structures as you make your longitudinal incision and try to keep them with the body wall. Avoid cutting deeper than the body wall. Much of the space in the hemocoel is filled with the amorphous white fat body in addition to tracheae, gut, and muscles. It will be necessary to detach some of the fat body from the body wall in order to pin the latter. Extend the incision anteriorly to the posterior border of the head capsule but do not cut into the capsule.
The body cavity opened by this incision is the perivisceral sinus, a division of the hemocoel (Fig. 6, 16-7). It is filled with blood (hemolymph). The much smaller pericardial sinus, which is also filled with blood, surrounds the heart and has been pinned aside with the body wall.
Hemal System
The insect hemal system consists of a dorsal tubular blood vessel extending the length of the body immediately under the dorsal midline and enclosed in the pericardial sinus. Posteriorly, for most of the length of the abdomen, the vessel is the muscular, pulsatile, and segmentally ostiate heart. The vessel continues anteriorly through the thorax to the head as the non-contractile aorta.
Look for the heart in the pericardial sinus attached to the body wall and pinned to the wax of the dissecting pan on the left side of your specimen (Fig. 5, 6). It may still be beating weakly with peristaltic waves passing from posterior to anterior. The heart is a transparent, colorless tube on the dorsal midline. It would be difficult to see were it not surrounded by greenish-brown tissues of the pericardial sinus. Some of the cells in this tissue are nephrocytes. A white band of fat body lies on either side of the pericardial sinus and helps define it visually. Large, translucent, strap-like longitudinal body wall muscles lie on either side of the heart and are reminiscent of the continuous muscle layers of an annelid rather than the individual muscles of an arthropod. Individual muscles are also present.
Fat Body
In Ceratomia the fat bodies are conspicuous in the perivisceral sinus as amorphous, flocculent, white tissue attached to the body wall and extending throughout the hemocoel. Fat bodies are conspicuous in the perivisceral sinus as seemingly amorphous white or yellow leafy sheets attached to the body wall and extending throughout the hemocoel. Note the tracheae extending to the fat bodies.
The fat body is a large, multipurpose, mesodermal organ consisting of various cells involved in intermediary metabolism, protein synthesis, and storage of lipids, carbohydrates, proteins, uric acid, and energy. In most insects the chief period of feeding, growth, and energy storage is as juveniles for adults feed relatively little or not at all. The energy accumulated by juveniles is stored in the fat body and later used to support the activities, including dispersal and reproduction, of the imago.
The system typically consists of a peripheral fat body forming a layer attached to the body wall and a perivisceral fat body associated with the gut wall as well as smaller concentrations with specific organs. The morphology of the fat bodies, only one or two cells thick, exposes a large surface area to the hemolymph and places all cells in contact with it for the exchange of materials.
Digestive System
The digestive system lies on the ventral midline where it is associated with the extensive white fat body and abundant tracheal tubes. The salivary (silk) glands and Malpighian tubules are also close to the gut and should be protected from damage. The gut consists of anterior ectodermal foregut, middle endodermal midgut, and posterior ectodermal hindgut, which may themselves be regionally specialized. Note the numerous branches of the visceral trachea extending to the gut.
Figure 5. Internal anatomy of a generalized caterpillar viewed from the left. Two of the three left side Malpighian tubules have been truncated for clarity. Redrawn and modified from Snodgrass (1935). Lepid64L.gif
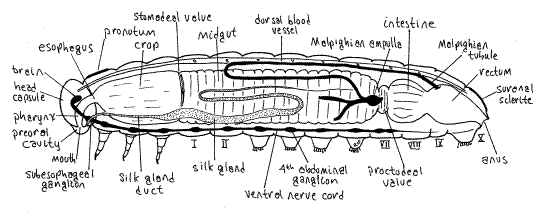
The foregut of Ceratomia, which is lined by cuticle, consists of mouth, pharynx, esophagus, and crop. Its chief function is food storage. The pharynx and esophagus are of small diameter and are largely enclosed within the head capsule. You have already seen the mouth. The esophagus can be seen emerging from the capsule where it widens to become the crop at the anterior end of prothorax (Fig. 5). Both have thin transparent walls and the crop is completely surrounded by a part of the fat body, making it appear to be white. The crop is a storage chamber occupying most of the space in the thoracic perivisceral sinus. It is probably full of recently ingested food particles cut from the host plant by the mandibles.
The midgut diameter is about the same as that of the crop and it occupies most of the anterior abdomen from segment 1 posteriorly to about segment 7. Its role is the secretion of digestive enzymes, hydrolysis of food, and absorption of food molecules after hydrolysis. Unlike the crop, its walls are thick and opaque. Remove the fat body from a portion of the midgut in the anterior abdomen to improve your view but do not damage the salivary glands or Malpighian tubules. The two salivary glands are long, clear, unbranched tubes whereas the Malpighian tubules, which are also tubes, are minutely branched and resemble long bottlebrushes (Fig. 5). Abundant tracheae radiate from each spiracle to the nearby region of the midgut. These are part of the visceral tracheal system. Avoid damaging the tracheae.
The midgut narrows abruptly in abdominal segment 6 to form the proctodeal valve (pyloric valve) which separates midgut and hindgut. The hindgut, which is cuticularized like the foregut, consists of a short intestine (anterior intestine) in segments 6-8, a short rectum (posterior intestine) in segments 9-10, and the anus, which you have already seen. The hindgut is responsible for reclamation of water and salts and formation and storage of fecal pellets. The intestine and rectum are separated from each other by a constriction. Later you will open the gut.
A pair of glands opening on the labium and appropriately known as the labial glands function in most insects as salivary glands and secrete saliva with hydrolytic enzymes adapted for the diet of the species. Such labial glands are sometimes referred to as salivary glands. In some taxa, most notably Lepidoptera (butterflies and moths), Trichoptera (caddisflies), and Hymenoptera (bees, ants, and wasps), the larval labial glands secrete, not saliva, but a proteinaceous silk used for a variety of purposes and are known as silk glands. In addition, caterpillars have a pair of mandibular glands, opening on the mandibular segment, that secrete saliva whose enzymes assist in the digestive process. The mandibular glands probably will not be seen.
In Ceratomia each silk gland is a long, unbranched, colorless, translucent tube lying to the right or left of the gut in the perivisceral hemocoel. A common duct from the spinneret arises within the head capsule, where you cannot see it, and bifurcates to form the right and left silk gland ducts (Fig. 5). These narrow, transparent ducts can be seen emerging laterally from the foramen magnum to enter the thorax. The ducts are small in diameter but quickly expand to become the silk glands. Each gland extends the length of the trunk to the posterior abdomen. Note the tracheae extending to the glands.
Although the true salivary glands do not secrete saliva, caterpillars also have a pair of mandibular glands and these do secrete saliva whose enzymes assist in the digestive process. The mandibular glands probably will not be seen.
Respiratory System
A typical insect respiratory system of interconnected tracheae is present in Ceratomia larvae and its major features can be seen by looking at a spiracle from inside the worm. The left first abdominal spiracle is probably the best to use for this purpose but any clearly visible, undamaged spiracle with intact tracheae can be used. Because of the position of our initial incision, the tracheae are less likely to have been cut on the left side. Some of the tracheae of your immersed specimen may still contain air. This renders them white or silver and makes them easier to see.
Tracheae arise from each spiracle and branch dendritically (tree-like) to supply nearby tissues and organs with oxygen and remove carbon dioxide (Fig. 6, 21-10). Each spiracle is associated with sunbursts of tracheae radiating outward and with unbranched longitudinal tracheal trunks connecting adjacent spiracles. The sunbursts supply nearby tissues with oxygen whereas the longitudinal trunks connect adjacent spiracles to form a single continuous system. The hemal system plays no role in oxygen transport in insects and the tracheal system ramifies throughout the body to provide oxygen to all tissues. During your dissection you have probably noticed the pervasiveness of the tracheal system.
Figure 6. Diagrammatic cross section of a generalized insect abdominal segment. Redrawn from Snodgrass (1935). Lepid65L.gif
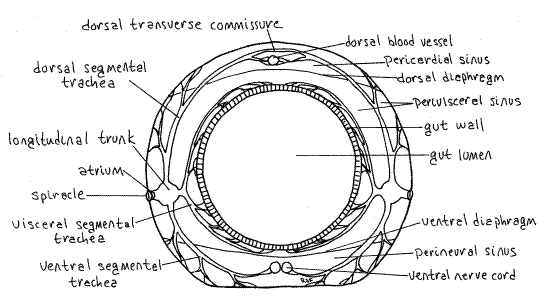
In a typical insect tracheal system, each spiracle opens into a short atrium that joins with one of the two lateral longitudinal trunks, one on the right and one on the left. Each trunk extends along a row of spiracles, either right or left, and connects them. From the atrium arise three major segmental tracheal systems, each with many, many branches. Together these form the sunbursts of tracheae mentioned earlier. The dorsal segmental trachea and its many branches extend to the dorsal body wall and heart musculature. The visceral segmental tracheasends branches to the gut, fat body, silk glands, and, in adults, to the gonads and gonoducts. The ventral segmental trachea supplies the ventral body wall musculature and nerve cord with oxygen.
In some segments the right and left dorsal segmental tracheae join each other across the dorsal midline to form a dorsal transverse tracheal commissure. Similarly some ventral segmental tracheae form ventral transverse commissures. Additional longitudinal trunks may be associated with the gut and dorsal body wall. These are smaller than the lateral longitudinal tracheal trunks. Note that, because of the longitudinal and transverse connections, the tracheae form a single interconnected system so that all parts of the body, whether or not they have spiracles, are supplied with tracheae and oxygen. The head and some thoracic segments, for example, lack spiracles but nevertheless are provided with oxygen by the system.
" Use fine scissors to remove a piece of a longitudinal trunk and make a wet mount with it. Examine the preparation with 100X, then 400X of the compound microscope. Note the chitinous rings, known as taenidia (Fig 21-10A), that reinforce the walls of the trachea and hold it open, much like the cartilaginous rings that hold your trachea open.
Excretory System
The excretory system includes nephrocytes and Malpighian tubules in the hemocoel. Lepidopteran larvae and adults have six Malpighian tubules, three arising on each side, right and left, of the midgut-hindgut junction in the vicinity of the proctodeal valve (Fig. 5, 16-9). On each side the cluster of three arises from a common ampulla evaginated from the gut but the connections with the gut are difficult to demonstrate in gross dissection. The tubules emerge from the gut at about the level of abdominal segment 6 and loop back and forth in the perivisceral hemocoel, making them difficult to count. To further complicate the pattern in Lepidoptera, the distal ends of the six tubules enter the connective tissue of the rectum wall and end there, out of sight, and there are thus no free ends of the tubules. For most of their length the tubules bear numerous short diverticula which make them look like long bottle brushes (Fig. 7). The tubules are supplied with tracheae.
Nephrocytes (Fig 16-7) are scattered throughout the hemocoel but most are concentrated in the dorsal diaphragm surrounding the heart. Nephrocytes are thought to be storage kidneys that absorb and sequester a variety of particles and dyes but they do not phagocytose bacteria.
Figure 7. A short section of a Malpighian tubule of Ceratomia catalpae. Lepid60L.gif

Digestive System Interior
" Using fine scissors, make a longitudinal, middorsal incision along the entire length of the exposed gut to open its lumen, which is probably filled with small pieces of catalpa leaf. In the midgut the food mass of leaf particles is enclosed in a thin transparent peritrophic membrane (Fig 21-9). Cut through the peritrophic membrane and use a Pasteur pipet to remove the leaf fragments and the membrane to improve your view of the gut lumen. The foregut (crop) is separated from the midgut by an inconspicuous stomodeal valve (cardiac valve), which inCeratomia, is manifest as a yellow ring and slight thickening of the gut wall (Fig. 5). In many insects the valve is more elaborate.
The peritrophic membrane is secreted by cells in the vicinity of the stomodeal valve. Note that there is no peritrophic membrane anterior to the foregut-midgut junction. The peritrophic membrane is secreted by the midgut epithelium to enclose the food mass. It is a porous mesh with the microvilli of the midgut epithelium protruding through its pores.
The midgut walls are opaque due to the thick, yellowish mucosal epithelium which lines the lumen. This epithelium is secretory and absorptive. Observe the lining of the midgut with about 30X of the dissecting microscope. The brush border of microvilli of the absorptive mucosal epithelium is strikingly visible (in living specimens) as a greenish-blue iridescence caused by the microvilli acting as a diffraction grating. Posteriorly the midgut epithelium is much thicker and colored dark brown. This region is also microvillar. A short segment of midgut without the brown microvillar epithelium connects the midgut with the hindgut.
The midgut and hindgut are separated by a thick, muscular proctodeal valve. The walls of the intestine and rectum are marked by thick longitudinal folds, or rugae. Find the anus from inside the rectum.
Nervous System
Ceratomia exhibits a, largely uncondensed and uncephalized nervous system typical of primitive insects. Such a system includes the tripartite brain, or supraesophageal ganglion, consisting of proto-, deuto- and tritocerebrum and the ventral nerve cord consisting of segmental ganglia joined by paired longitudinal connectives. The brain innervates the eyes (protocerebrum), antennae (deutocerebrum), and labrum (tritocerebrum). The tritocerebrum is connected with the double longitudinal ventral nerve cord by a pair of circumesophageal connectives. Immediately posterior to the connectives is the subesophageal ganglion consisting of the fused ganglia of the mandibles, maxillae, and labia. The segmental ganglia of the thorax and abdomen remain independent of each other and are spaced along the nerve cord.
" Cut the tracheae on the right side of the gut, move the gut to the left, and pin it so the ventral midline of the body wall is revealed. Remove fat bodies as necessary to reveal the ventral midline of the thorax and abdomen being careful that you do not damage the nerve cord on that midline. Do not attempt to remove the small ventral fat bodies associated with the nerve cord. The white segmental ganglia and nerve cord are easy to see on the midventral body wall after removal of the gut. For most of its length the longitudinal connectives are coalesced to form a single nerve cord.
The brain and subesophageal ganglion are in the head capsule but can be seen by lifting the capsule and looking into the foramen magnum using 20X (Fig 16-11, 21-7). Thesubesophageal ganglion, which serves the mandibles, maxillae, and labium, is in the posterior head capsule ventral to the gut (Fig. 5). The brain is dorsal to the gut. The subesophageal ganglion is connected by a coalesced pair of short connectives with the first thoracic ganglion (Fig 16-11), which is very close to it and just outside the head capsule in the prothorax. Thesecond thoracic ganglion is well separated from the first and connected to it by a pair of long connectives that are coalesced for part of their length and fused for part. The third thoracic ganglion is far posterior to the second and is connected with it by a pair of long connectives, also partly fused and partly separated.
Abdominal ganglia 1-7 are widely spaced along the length of the abdomen and are connected by two coalesced connectives, which form an seemingly single ventral nerve cord (Fig. 5, 6).
Abdominal ganglion 8 is very close (touching) to the seventh ganglion and their connectives are very short. Several nerves radiate posteriorly from the eighth ganglion but the nerve cord ends with the eighth.
Examine one of the ganglia, such as the first abdominal, with 40X and find the pair of inconspicuous segmental nerves exiting the ganglion laterally and extending to the periphery. Note that the nerve cord, ganglia, and nerves are supplied with tracheae. Do not confuse segmental nerves with tracheae
Note that ventral longitudinal body wall muscles, similar to the dorsal muscles seen beside the heart, lie beside the nerve cord.
Reproductive System
The reproductive system of caterpillars is rudimentary and will develop later, in the pupa during metamorphosis. Small gonads, however, are present in late instar larvae and spermatogenesis occurs in these larvae and in the pupa. Eggs develop in the pupa. Development of both internal and external genitalia occurs during metamorphosis in the pupa.
*Hyphenated figure call-outs, such as this one, refer to figures in Ruppert, Fox, and Barnes (2004). Those without hyphenation refer to figures embedded in this exercise.
References
Borror DJ, Triplehorn CA, Johnson NF . An introduction to the study of insects, 6 th ed. Saunders College Publishing, Philadelphia. 875pp.
Chapman RF. 1998. The insects, Structure and function, 4 th ed. Cambridge Univ. Press, Cambridge. 769 pp.
Comstock JH. 1930. An introduction to entomology. Comstock Pub., Ithaca. 1044 pp.
Hyche LL. 1994. The Catalpa Sphinx. Auburn University.
www.ag.auburn.edu/dept/entplp/bulletins/caltalpasphinx/catalpasphinx.htm
Ness J. 2002. Center for Insect Science.
http://cis.arl.arizona.edu/PERT/people/Ness/Research.htm
Oehlke W. Undated. Ceratomia catalpae.
www.silkmoths.bizland.com/ccatalpa.htm
Ross HH. 1965. A textbook of entomology, 3 rd ed. John Wiley & Sons, New York. 539pp.
Ruppert EE, Fox RS, Barnes RB. 2004. Invertebrate Zoology, A functional evolutionary approach, 7 th ed. Brooks Cole Thomson, Belmont CA. 963 pp.
Snodgrass RE . 1935. Principles of insect morphology. McGraw-Hill, New York. 667 pp.
Supplies
1 1iving, freshly collected, late instar Ceratomia catalpae caterpillar
1 small (anchovy tin) wax-bottom dissecting pan
1 dissecting microscope
1 compound microscope (can be shared by several students or the entire class)
1 microscope slide and coverslip
1 microdissecting forceps
1 microdissecting (iridectomy) scissors