Invertebrate Anatomy OnLine
Callinectes sapidus ©
Blue Crab
with notes on Cancer
30may2007
Copyright 2001 by
Richard Fox
Lander University
Preface
This is one of many exercises available from Invertebrate Anatomy OnLine , an Internet laboratory manual for courses in Invertebrate Zoology. Additional exercises can be accessed by clicking on the links to the left. A glossary and chapters on supplies and laboratory techniques are also available. Terminology and phylogeny used in these exercises correspond to usage in the Invertebrate Zoology textbook by Ruppert, Fox, and Barnes (2004). Hyphenated figure callouts refer to figures in the textbook. Callouts that are not hyphenated refer to figures embedded in the exercise. The glossary includes terms from this textbook as well as the laboratory exercises.
Systematics
Arthropoda P, Mandibulata, Crustacea sP, Eucrustacea, Thoracopoda, Phyllopodomorpha, Ostraca, Malacostraca C, Eumalacostraca, Caridoida, Decapoda O, Brachyura sO, Brachyrhyncha iO, Portunoidea SF , Portunidae F (Fig 16-15, 19-67, 19-90)
Arthropoda P
Arthropoda, by far the largest and most diverse animal taxon, includes chelicerates, insects, myriapods, and crustaceans as well as many extinct taxa such as Trilobitomorpha. The segmented body primitively bears a pair of jointed appendages on each segment. The epidermis secretes a complex cuticular exoskeleton which must be molted to permit increase in size. Extant arthropods exhibit regional specialization in the structure and function of segments and appendages but the ancestor probably had similar appendages on all segments. The body is typically divided into a head and trunk, of which the trunk is often further divided into thorax and abdomen.
The gut consists of foregut, midgut, and hindgut and extends the length of the body from anterior mouth to posterior anus. Foregut and hindgut are epidermal invaginations, being derived from the embryonic stomodeum and proctodeum respectively, and are lined by cuticle, as are all epidermal surfaces of arthropods. The midgut is endodermal and is responsible for most enzyme secretion, hydrolysis, and absorption.
The coelom is reduced to small spaces associated with the gonads and kidney. The functional body cavity is a spacious hemocoel divided by a horizontal diaphragm into a dorsal pericardial sinus and a much larger perivisceral sinus. Sometimes there is a small ventral perineural sinus surrounding the ventral nerve cord.
The hemal system includes a dorsal, contractile, tubular, ostiate heart that pumps blood to the hemocoel. Excretory organs vary with taxon and include Malpighian tubules, saccate nephridia, and nephrocytes. Respiratory organs also vary with taxon and include many types of gills, book lungs, and tracheae.
The nervous system consists of a dorsal, anterior brain of two or three pairs of ganglia, circumenteric connectives, and a paired ventral nerve cord with segmental ganglia and segmental peripheral nerves. Various degrees of condensation and cephalization are found in different taxa.
Development is derived with centrolecithal eggs and superficial cleavage. There is frequently a larva although development is direct in many. Juveniles pass through a series of instars separated by molts until reaching the adult size and reproductive condition. At this time molting and growth may cease or continue, depending on taxon.
Mandibulata
Mandibulata is the sister taxon of Chelicerata and in contrast has antennae on the first head segment, mandibles on the third, and maxillae on the fourth. The brain is a syncerebrum with three pairs of ganglia rather than the two of chelicerates. The ancestral mandibulate probably had biramous appendages and a J-shaped gut, posterior-facing mouth, and a ventral food groove. The two highest level mandibulate taxa are Crustacea and Tracheata.
Crustacea sP
Crustacea is the sister taxon of Tracheata and is different in having antennae on the second head segment resulting in a total of 2 pairs, which is unique. The original crustacean appendages were biramous but uniramous limbs are common in derived taxa. The original tagmata were head but this has been replaced by head, thorax, and abdomen or cephalothorax and abdomen in many taxa. Excretion is via one, sometimes two, pairs of saccate nephridia and respiration is accomplished by a wide variety of gills, sometimes by the body surface. The nauplius is the earliest hatching stage and the naupliar eye consists of three or four median ocelli.
Eucrustacea
Eucrustacea includes all Recent crustaceans except the remipedes. The taxon is characterized by a primary tagmosis consisting of heat, thorax, and abdomen although the derived condition of cephalothorax and abdomen is more common. Eight is the maximum number of thoracic segments.
Thoracopoda
In the ancestral thoracopod the thoracic appendages were turgor appendages used for suspension feeding in conjunction with a ventral food groove. Such appendages and feeding persist in several Recent taxa but have been modified in many others.
Phyllopodomorpha
The compound eyes are stalked primitively although derived sessile eyes occur in many taxa.
Malacostraca C
Malacostraca includes most of the large and familiar crustaceans such as crabs, shrimps, lobsters, crayfish, isopods, amphipods, and others. Primitively the trunk consists of 15 segments, eight in the thorax and seven in the abdomen but in most Recent species the abdomen has only six segments (Fig 19-19). The female gonopores are on the eighth thoracic segment, male on the sixth.
Decapoda O
The largest and most familiar crustaceans belong to Decapoda. The 10,000 species of crabs, shrimps, crayfishes, lobsters, and their relatives are decapods. The first three segments of the decapod thorax are fused with the head to form a cephalothorax and their appendages are maxillipeds. The remaining five pairs of thoracic appendages bear simple or chelate walking legs. The resulting ten legs accounts for the name “decapod”. A large carapace extends posteriorly from the head and is fused dorsally with all eight thoracic segments. Laterally the overhang of the carapace encloses the branchial chamber with the gills. The most primitive decapods (shrimps, lobsters, and crayfishes) have well developed abdomens whereas the most derived taxa (true crabs in Brachyura) have reduced, almost vestigial, abdomens (Fig 19-24).
Laboratory Specimens
The blue crab, Callinectes sapidus , is a large crab of shallow waters along the east coast of North and South America. Callinectes belongs to the swimming crab family, Portunidae, most of whose members have large oarlike hind legs that are used for swimming. This exercise was written for Callinectes but any portunid crab, such as Portunus or Ovalipes, will serve equally well. Parenthetical comments refer to a similar genus, Cancer, in the family Cancridae.
The dissection can be performed on preserved, frozen, or living anesthetized specimens. Seafood markets sometimes offer steamed crabs and even these can be used for a laboratory exercise of external anatomy.
Living crabs can be anesthetized in about 20 minutes by immersion in carbonated water or chloroform-saturated seawater. The inhalant aperture of the gill chamber must be immersed in the liquid in order for anesthetization to occur.
Freezing for about 40 minutes will kill the crabs and provide specimens suitable for dissection. Active specimens must be handled with caution as they are quick, willing, and able to inflict painful wounds. With experience, they can be handled safely and confidently by firmly grasping, with thumb and forefinger, the base of one of the two swimming legs. Don’t try this if you don’t know what you are doing. Blue crabs really hurt. The pincers of unanesthetized crabs should be inactivated with small rubber rings made by cutting cross sections of surgical rubber tubing.
Natural History
Callinectes sapidus supports an important fishery along the east coast of North America and is an important commercial species. Blue Crabs are notoriously fast, pugnacious, evil-tempered, and voracious. They are omnivores that feed on a variety of living and dead animal and plant material.
Because of its economic importance, much is known of its biology. Mature females spawn in high salinity water near the mouths of estuaries and the planktonic larvae make their way into the estuary to lower salinity upstream waters. They settle to the bottom and begin a benthic existence, molting, growing, and moving downstream to higher salinities. Mating occurs only once in the life of the female and afterwards she migrates to the mouth of the estuary and the nearby ocean. Here she spawns, using stored sperm to fertilize her eggs. Planktonic larvae hatch from the eggs and begin the journey back into the estuary.
Callinectes exemplifies the most derived malacostracan morphology and shows several departures from that of the ancestral crustaceans. Notable among these are the extreme reduction of the abdomen, the cephalization of the nervous system, and the shortening, broadening, and flattening of the body. As you conduct the dissection, think about the morphology of a more primitive malacostracan such as a shrimp, lobster, or crayfish and consider how it differs from that of crabs.
External Anatomy
Body and Tagmata
Examine the crab and note that, unlike more primitive decapods such as shrimps and crayfish, the body is short, very wide, and dorsoventrally depressed (Fig 1, 19-31). The ancestral crustacean tagmata of head, thorax, and abdomen have been reorganized into cephalothorax, pereon, and abdomen but the abdomen is reduced and almost vestigial. The malacostracan head has five segments, the thorax eight, and the abdomen six. The head and first three thoracic segments are fused to form a new tagma, the cephalothorax leaving five free thorax segments as the pereon. The reduced abdomen is folded beneath the thorax and is not visible dorsally. Most of the crab body is cephalothorax and pereon which are covered dorsally by a large, hard, shield-like carapace. The carapace is an outgrowth of the most posterior head segment.
Head and Cephalothorax
In decapods, the first three thoracic segments are fused with the five head segments and their appendages are maxillipeds. Together the head and first three thoracic segments form thecephalothorax. Each thoracic segment of the cephalothorax bears a pair of maxillipeds but there are few external indications of the original segmentation. Dorsally and laterally the head and entire thorax are covered by the carapace.
Thorax
The thorax consists of eight segments, or thoracomeres, of which the first three are part of the cephalothorax. The five posterior thoracomeres are not fused with the head although dorsally they appear to be because all are covered by the carapace. Ventrally, however, the five segments can be seen to be independent of the cephalothorax (Fig 2, 19-31). The five independent thoracic segments are known collectively as the pereon and their appendages are pereopods. Each of these segments is a pereomere. The pereopods are the five pairs of large, obvious walking legs (Fig 1, 19-31). The name Decapoda (“ten feet”) alludes to these ten appendages.
Ventrally, where they are not covered by the carapace, the thoracomeres are easy to distinguish (Fig 2, 19-31). The first three, which bear maxillipeds, are fused to each other and to the head but the posterior five, numbered 4-8 in Figure 2, are clearly independent of each other. The ventral surface of a typical arthropod segment is covered by an exoskeletal plate known as asternite and those of the thoracomeres form the ventral surface of the thorax (Fig 2, 19-31).
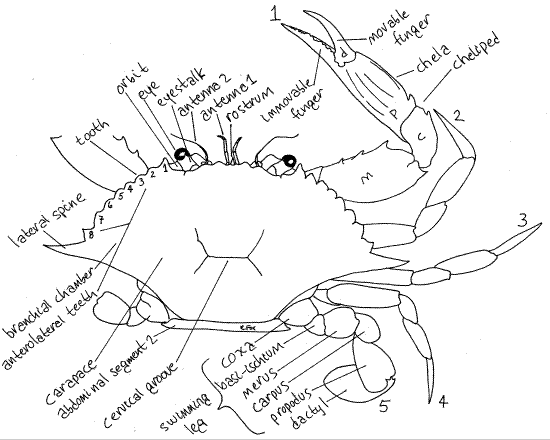
Figure 1. Dorsal view of the blue crab, Callinectes sapidus. The pereopods of the left side have been omitted. The anterolateral teeth are numbered 1-8. The pereopods are numbered 1-5. m = merus, c = carpus, p = propodus, d = dactyl. Crab20La.gif
Carapace
The carapace has two long lateral spines and several strong teeth on each anterolateral margin (Fig 1). The posterior margin of the carapace is smooth or minutely beaded. The lateral extensions of the carapace, known as branchiostegites, enclose large branchial chambers which house the gills (Fig 19-36, 19-3A).
On the anterior edge of the carapace, on each side of the midline are two shallow, notched excavations. These are the orbits, from which the eyestalks protrude (Fig 1). Each has acompound eye at its distal end. Anteriorly the cephalothorax bears a small anterior, median process, the rostrum (Fig 1).
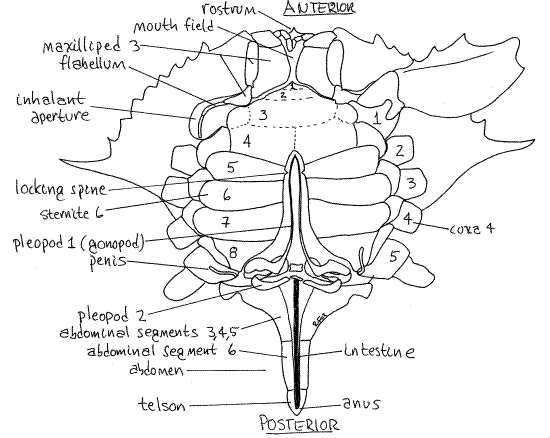
Figure 2. Ventral view of a male blue crab. The sternites of the thoracomeres are numbered 1-8. The left coxae of the pereopods are numbered 1-5. The abdomen is extended to reveal the abdominal appendages and penis. Crab21La.gif
Crossing the midline of the carapace, just posterior to the middle, is a short, shallow, transverse groove (inconspicuous in Cancer). This is the cervical groove and it is the cross-bar of an H-shaped set of grooves (Fig 1). It marks the approximate division between head and thorax.
Abdomen
On the ventral surface locate the abdomen (Fig 2) flexed beneath the thorax (Fig 19-31). The abdomen is also called the pleon, its segments are pleomeres, and its appendages arepleopods. In true crabs (i.e. Brachyura, such as Callinectes and Cancer) the abdomen is a small segmented structure whose shape varies with sex and maturity. In mature females it is broad with convex sides and covers most of the posterior ventral surface of the thorax. In immature females the abdomen is a nearly equilateral triangle whereas the abdomen of males is very narrow although it has a broad base. Determine the sex of your specimen from the shape of the abdomen.
Extend the abdomen so it is no longer flexed but points posteriorly from the thorax as it would in a crayfish or shrimp. In dorsal view most of its segments are easily seen and can be counted, especially in females. The small, triangular, terminal portion is the telson, which is not a true segment. Most malacostracans have six abdominal segments plus the terminal telson. In female blue crabs the six segments are independent of each other and five of them are visible, the first being hidden by the carapace. In males, segment 1 is hidden under edge of the carapace, segment 2 is visible and wide, and 3, 4, and 5 are visible but fused together and narrowed posteriorly (Fig 2). Segment 6 is separate, slender, and has the telson attached to its end. (The segments differs in other genera).
The transparent, membranous intestine runs along the ventral midline of the abdomen, under the thin membranous ventral exoskeleton, and terminates at the anus on the telson (Fig 2). It may be filled with dark feces in which case it is easier to see. Press its posterior end with a probe to extrude feces from the anus, thereby confirming its position.
On the ventral surface of the thorax is a median, longitudinal groove hidden by the abdomen. The abdomen of the male occupies this groove and in females the gonopores are in its walls. The female gonopores are large triangular openings in the sternites of the sixth thoracic segment, in line with the third pair of legs. Male gonopores are located at the tip of the inconspicuous penis on the last leg and will be seen later.
Appendages
Study the appendages without removing them from the animal. Each section of an arthropod limb is known as an article. The term segment is reserved for the modular divisions of the body.
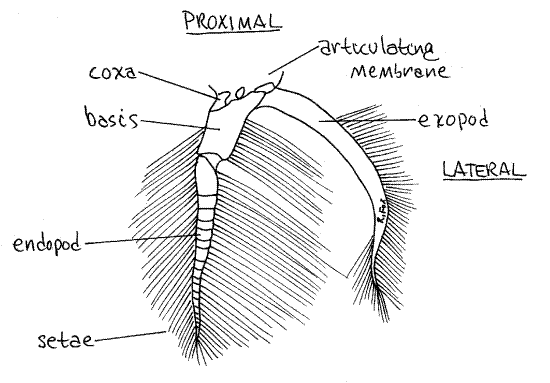
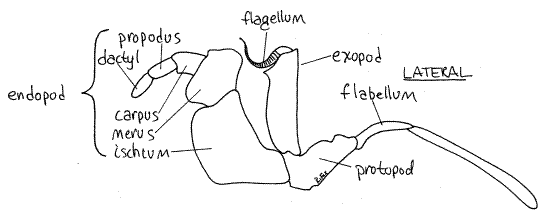
The basic crustacean appendage is biramous. The proximal article, the one that articulates with the body, is the protopod. In many instances the protopod is divided into two articles, the coxa and basis. From the protopod (or basis if the protopod is divided) arise two branches, or rami. The lateral ramus is the exopod and the medial is the endopod. If one ramus is absent, the appendage is uniramous. If both are present, it is biramous. Any additional structures on the lateral side the limb are exites, on the medial side they are endites. Finally, an exite on the base of the appendage is an epipod.
Although appendages are numbered from anterior to posterior it is easier to study them in reverse order, from posterior to anterior. Begin with the pleopods, or abdominal appendages, and work your way forward through the pereopods, maxillipeds, and mouthparts, to end with the antennae. Brachyuran crabs have no uropods.
Extend the abdomen again, look at its ventral surface, and find the abdominal appendages (pleopods). Be sure you see and study both sexes.
Pleopods
Like the abdomen itself, the pleopods are sexually dimorphic.
Male
Males have only two pairs of pleopods and they are located anteriorly on the abdomen, on segments 1 and 2 (Fig 2). Both function in the transfer of sperm to the female during copulation. They are hidden under the flexed abdomen, which must be extended to reveal them. The long, curved, tubular first pleopod is the gonopod. It, not the penis, is the intromittent organ used to deliver spermatophores to the female gonopore. The second pleopod is much smaller and functions as a piston to push spermatophores through the hollow core of the gonopod.
Female
Females have paired biramous pleopods on abdominal segments 2-5 and, as in the male, they are hidden under the flexed abdomen which must be extended to reveal them. The first article, or coxa (Fig 3), of a female pleopod is attached to the body by a soft and flexible articulating membrane. The coxa is small and poorly calcified but the next article, the basis, is large and conspicuous. Two rami, the exopod and endopod, arise from the basis. After release from the gonopores, the eggs attach to the long setae of the pleopods where they are ventilated by movements of the abdomen and the pleopods.
Figure 3. The pleopod of abdominal segment 5 of a female crab. Crab22L.gif
Pereopods
The five pairs of pereopods, or walking legs, of the posterior thorax lack exopods and thus are uniramous. They are slender stenopods consisting solely of the endopod and protopod. They have no exopod. In almost all swimming crabs (Portunidae) pereopod 5 is a broad, oarlike swimming leg (Fig 1, 19-31). (The fifth pereopod of Carcinus maenas, although a portunid, is not modified for swimming.)
Find the characteristic seven articles of a typical thoracic malacostracan endopod (Fig 1). The short, proximal coxa articulates with the body (Fig 2). The next two articles, the basisand ischium, are fused together to form the basischium. The suture marking the line of fusion is visible. The basischium is followed successively by the merus, carpus, propodus, anddactyl. The dactyl and propodus of pereopod 5 are both flattened to form the paddle.
In males the gonopore is located at the tip of a long, thin, limp, transparent, colorless penis on the proximal edge of the coxa of the fifth pereopod (Fig 2). (The penis of Cancer is a short, blunt papilla). The penis fits into the groove of the gonopod to which it delivers sperm. The gonopods, not the penis, are the intromittent organs.
Pereopods can be voluntarily autotomized (= self cut) to escape predation, reduce blood loss from a distal wound, or in response to physiological stress. A special fracture plane is located approximately at the line of fusion of the basis and ischium, where each leg can be shed with a minimum of bleeding and trauma.
Pereopods 2-4 resemble pereopod 5 except that their distal articles are not oarlike (Fig 1). Pereopod 1 is the cheliped and the pincer at its distal end is the chela. The cheliped is larger and more robust than the other pereopods and is constructed so that the dactyl is a movable finger that opposes an immovable finger protruding from the propodus. This arrangement creates a prehensile chela. Note the teeth on the fingers.
Note the slight asymmetry of the two chelipeds. The left, or cutter cheliped, is smaller and its teeth are a little smaller and sharper. The right, or crusher cheliped, is a bit larger and has larger and slightly more rounded teeth. This dimorphism may be reversed in some individuals and it is more pronounced in many other crab species. (There is little difference in the two chelipeds of Cancer irroratus)
The large opening in the carapace dorsal to the coxa of the cheliped is the inhalant aperture leading into the branchial chamber (Fig 2) where the gills are located.
If your crab is missing any of its legs, they were probably deliberately autotomized by the crab. If so, the end of the stump of the leg will be cleanly sheared at the fracture plane and covered by a membrane. The membrane is penetrated by a small hole through which pass an artery and nerve (before autotomy). A one-way valve over this opening prevents loss of blood after autotomy. Autotomy avoids irregularly broken exoskeleton, and torn muscles. Regeneration of an autotomized leg begins before the next molt.
Maxillipeds
The three pairs of maxillipeds are the appendages of the first three thoracomeres. Unlike other thoracopods they are biramous. Their endopods are homologous to the endopods of the pereopods and are composed of the same seven articles.
The maxillipeds and other mouthparts overlie each other so only maxilliped 3 can be seen at present. After you study each appendage it should be moved aside to reveal the one beneath it (anterior to it). Do not detach the maxillipeds from the body.
Third Maxilliped
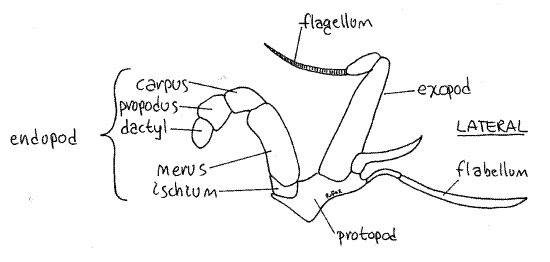
Look at the crab en face, with magnification as needed, and find the quadrate mouth field including the mouth, the area around it, and the mouthparts. The third maxillipeds cover the mouth field (Fig 2). They are the appendages of the third thoracomere and together they resemble a pair of doors protecting the mouth field and hiding the other mouthparts.
Move one of the third maxillipeds and note its mobility. It is attached to the body by its protopod (Fig 4). The protopod forms the medial border of the inhalant aperture and bears two rami. The endopod is the wider of the two rami and its distal three articles form a small palp. The endopod is composed of the large proximal ischium followed by the merus, carpus,propodus, and dactyl. The exopod consists of a long, narrow basal article and a multiarticulate flagellum used to groom the face. At the lateral corner of the protopod you will see a long setose flabellum extending through the inhalant aperture into the branchial chamber. If you move the maxilliped, the flabellum will move also, making it easier to recognize. The flabellum is used to clean the gills.
Figure 4. The third maxilliped. Crab23L.gif
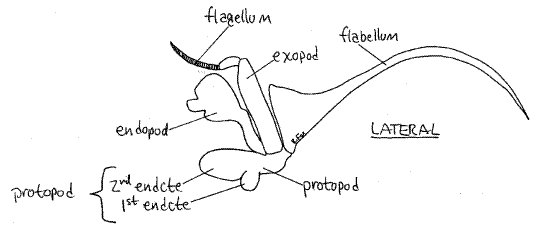
Second and First Maxillipeds
The second and first maxillipeds are similar to the third but are smaller (Figs 5, 6). They are the appendages of the second and first thoracomeres respectively. Like the third, they bear endopods, exopods, and lateral flabellae that extend into the branchial chamber.
Figure 5. The second maxilliped. Crab24L.gif
The two exhalant apertures, through which water exits the branchial chambers, are not so obvious as the inhalant aperture but can be seen lateral to the first and second maxillipeds. Water can exit these openings even when the third maxillipeds are closed over the mouth field.
Mouthparts
The remaining five pairs of appendages belong to the head segments and comprise three pairs of mouthparts and two pairs of antennae. Move the maxillipeds aside to reveal the mouthparts.
Figure 6. The first maxilliped. Crab25L.gif
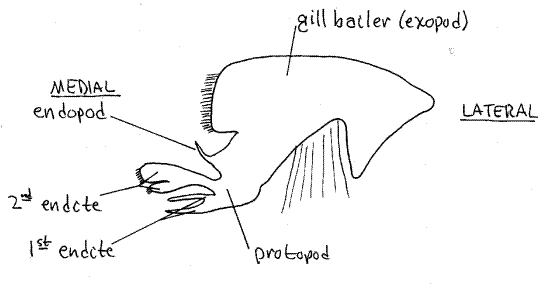
Second Maxilla
The posteriormost head appendage is the second maxilla (Fig 7) lying immediately anterior to the first maxilliped. It is small, complex, and more delicate than the maxillipeds. It has several parts, one of which is a large, lateral, flat, rectangular gill bailer, or scaphognathite, which projects through the exhalent aperture into the branchial chamber. The bailer, which generates the respiratory current through the branchial chamber, is the exopod of this appendage (Fig 19-38C). The protopod has two flat, setose, bilobed endites and a vestigial endopod between the bailer and the endites.
Figure 7. The second maxilla. Crab26L.gif
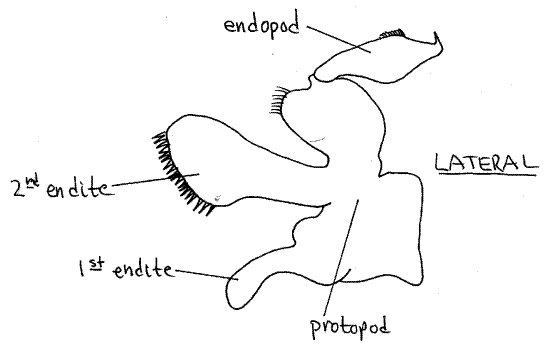
First Maxilla
The first maxilla (Fig 8) is even smaller and more delicate than the second. It has two endites and an endopod but no exopod.
Figure 8. The first maxilla. Crab27L.gif
Mandible
Anterior to the first maxillae are the large, hard mandibles (Fig 9). Each mandible consists of a heavily calcified protopod from which arises a small palp. The protopod is divided into a medial cutting surface and a large basal region for the insertion of the muscles that operate the mandible. Only the smooth, white cutting surfaces can be seen externally. Push the mandibles back and forth and watch their motion. They rotate on two movable articulations, or condyles, with the head skeleton and are adapted for cutting, rather than grinding, food.
Figure 9. The mandible. Crab28L.gif
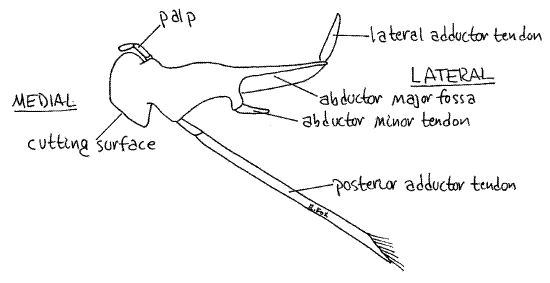
Push the two cutting surfaces apart and notice the soft protuberant upper lip; a transverse fold of the body wall. Ventral to the lip and between the mandibles, is the mouth. Gently insert your blunt probe into the mouth to confirm its location.
Antennae
Both pairs of antennae are small and may go unnoticed if they are folded out of sight under the anterior edge of the carapace (Fig 1).
Antenna 2
The lateral pair is the second antennae. In crabs they lack exopods and are uniramous (Fig 10). Each consists of a basal peduncle, of three articles, whose proximal article is fused with the carapace and bears a small, heavy, transverse ridge. Distally the peduncle bears a slender flagellum of many articles.
Figure 10. The first (right) and second (left) antenna of Callinectes similis. The peduncular articles of each are numbered. The inset is an enlargement of the base of antenna 2 showing the nephridiopore and its operculum. Crab29L.gif
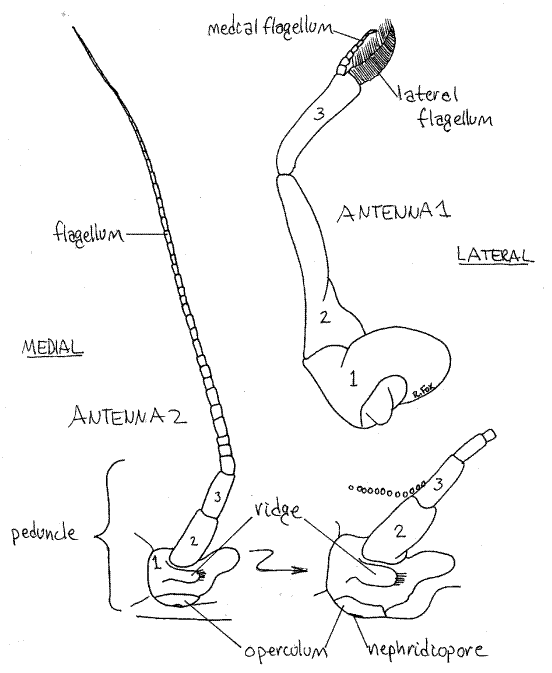
Find the short pronounced ridge running transversely across the first peduncle article (Fig 10). Below the ridge is a second, longer, ridge forming the dorsal border of the mouth field. The external opening of the nephridium, the nephridiopore, is located in the depression between these two ridges. The pore is covered by a movable calcified operculum. (In Cancer there are no transverse ridges but there is a tiny oval operculum covering the nephridiopore.) Insert the tip of a needle under the ventral edge of the operculum and lift it to demonstrate its mobility. In living animals urine may escape when the operculum is lifted. The operculum is best observed with magnification.
Antenna 1
The first antenna (Fig 10) consists of a triarticulate peduncle from which arise two short inconspicuous flagella. The basal article of the peduncle is broad and swollen and fits in a socket in the anterior body wall. It contains a statocyst for the detection of gravity (Fig 19-7B). The statocyst is an invagination of the exoskeleton containing a statolith resting on sensory setae. The basal peduncular article is not fused to the carapace. The first antennae fold on themselves and fit neatly into a recess in the carapace.
A small, pointed, median rostrum extends anteriorly between the bases of the two first antennae (Fig 1).
Eyestalks
The two short, thick eyestalks, which are not segmental appendages, are located on the anterior edge of the head in the orbits (Fig 1). A large compound eye, located at the end of each eyestalk is composed of hundreds of independent photoreceptive units, or ommatidia. The external manifestation of each ommatidium is its cuticular lens.
Internal Anatomy
" Turn the crab so its dorsal side is up. Insert the tip of a heavy scissors beneath the lateral, posterior edge of the carapace, dorsal to the coxa of the fifth leg, and make a cut around the periphery of the carapace on its dorsal surface (Fig 11). Be careful that you cut only the heavy calcified exoskeleton and not the organs beneath it. Keep your scissors about 5 mm from the edge of the carapace and cut completely around it. Use a scalpel to separate it (by scraping, not cutting) from the underlying tissues. Carefully remove the carapace, in pieces if necessary, with minimal disturbance to the underlying tissues.
The thin, dark body wall, which is little more than the epidermis, lies immediately beneath the carapace and as much of it as possible should be removed with the carapace. The exoskeleton and epidermis are the body wall, as there is no musculature, connective tissue, or peritoneum in it.
Notice two small, digitiform, calcareous processes on the inner surface of the carapace almost exactly in its center. These are apodemes for the origin of muscles running to the gut. These muscles must be disconnected to remove the carapace.
The hemocoel is the large space in which the viscera lie. The coelom is present only as small spaces associated with the gonads and nephridia.
Carefully remove most of the remaining epidermis (i.e. body wall) without damaging the underlying tissues. It may help to flood its surface with water to facilitate its removal. This is a tedious task but must be done with care as some of the crab's organs adhere tightly to it.
>1a. Make a wholemount of a small piece of epidermis being sure the tissue is flat and not folded. Examine the preparation with the compound microscope. The pigment in the body wall is contained in conspicuous chromatophores (Fig 19-44A,B). These are irregular, star-shaped multicellular organs containing red, blue, or white pigment granules. Red and black can be found easily but the white chromatophores are harder to see because the pigment is not so vivid.
The pigment can be dispersed into the lobes of the chromatophore to increase its visual effect or it can be concentrated in the center to minimize it. <
Preview
Make a preliminary examination of the hemocoel and its viscera to locate major structures for use as landmarks. If your specimen is a mature female, the orange ovaries may cover and obscure other structures. The smaller, white testes of the mature male do not obscure other structures. It may be necessary to remove the ovary (but nothing else) from one side in order to see the stomach and digestive ceca beneath.
The stomach (= proventriculus) is a large, bulging, transparent, thin-walled sac lying dorsally on the midline in the anterior thorax (Fig 11, 19-35). The digestive ceca are large, soft, amorphous, yellow or greenish organs occupying the periphery of the dorsal thorax. They may be completely obscured by the ovary in mature females. (The digestive ceca of Cancer are gray-brown and consist of abundant small, fingerlike papillae.)
Figure 11. Dorsal dissection of a mature male crab. The digestive cecum, gonads, and gills have been removed from the left side. Crab30La.gif
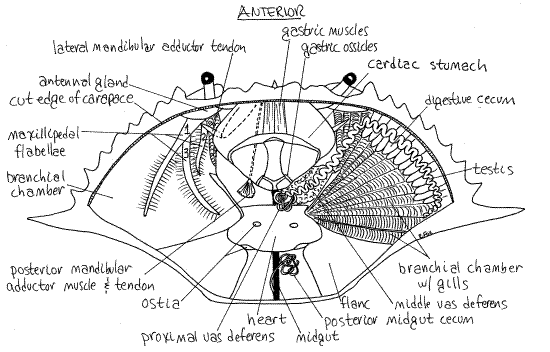
The large, triangular, firm, beige or greyish mass of gills occupies the branchial chamber in the space medial to the lateral spine (mostly posterior to the lateral spine in Cancer). The gills are sometimes called "dead man's fingers".
The triangular mass of gills is covered by a very thin, transparent membrane which you should avoid damaging. Posterior to each gill chamber is a heavy endoskeletal plate called theflanc that covers the powerful swimming muscles of pereopod 5. These muscles are the "backfin" crabmeat of the seafood industry. (In Cancer the skeletal plate enclosing the muscles of the last leg is not markedly larger than those of the other legs). The soft, white or gray heart lies on the midline posterior to the stomach and between the flancs.
A more careful study of the organ systems can now be undertaken. Systems are considered in the order in which they are exposed by the dissection. The internal organs of crabs have little connective tissue and are very delicate so that some organs appear shapeless and without definite structure.
Hemal System
The opaque white or gray heart (Figs 11, 12, 19-39) lies in a hemocoelic space known as the pericardial sinus. This unlined, blood-filled region in the hemocoel is not a coelom and should not be called a pericardium, although it often is. The heart has three pairs of large ostia, two dorsal and one lateral, through which blood enters the heart (Fig 11, 19-35). The heart is suspended by numerous elastic suspensory ligaments that run to the surrounding tissues. These ligaments are stretched during systole (contraction) and then return to their original length when the heart muscles relax during diastole. This restores the heart to its original size and volume and results in the influx of blood through one-way valves in the ostia into the heart lumen.
The heart of living specimens may be beating. Contractions of the heart pump blood anteriorly, ventrally, and posteriorly via a set of seven arteries (Fig 12, 19-39). The arteries are difficult to observe in fresh specimens but some, or all, of them are usually visible in preserved animals. The arteries branch repeatedly until, by the time they reach the tissues, their diameter is that of capillaries.
The blood leaves the capillaries and bathes the tissues. It then flows into the hemocoel, passes through the gills, and then drains back into the cardiac hemocoel. When the heart relaxes, the valves of the ostia open and admit blood to the heart lumen. Upon contraction the valves close and blood enters the arteries.
The blood, or hemolymph, contains the respiratory pigment hemocyanin, which is colorless when deoxygenated and pale blue when oxygenated. The pigment is in solution in the hemolymph.
>1b. If the heart is beating, inject a solution of toluidine blue in seawater through an ostium into its lumen and watch the dye enter the major arteries. Trace the route of the arteries as far as possible. <
Digestive System
The arthropod gut consists of an anterior foregut, middle midgut, and posterior hindgut. The foregut and hindgut are derived from ectoderm and are lined with exoskeleton that is shed with each molt. The midgut is an endodermal derivative and has no cuticular lining. The foregut comprises the mouth, esophagus, and stomach. The crab midgut consists of the tubular midgut itself and several diverticula including the digestive ceca and three midgut ceca. All digestion and absorption occur in the midgut and its derivatives. The hindgut consists of the tubular intestine, rectum, and anus.
The large yellowish digestive ceca (= midgut glands, digestive glands, or hepatopancreas) are diverticula from the midgut (Fig 11). They connect with the midgut near its junction with the stomach but the connections are difficult to observe. The digestive ceca extend along the anterior edge of the carapace, over the branchial chambers, and in the spaces around the heart and gonoducts. The ceca secrete hydrolytic enzymes into the stomach, absorb the products of hydrolysis, and store food reserves.
The stomach is the largest and most conspicuous part of the gut (Fig 11, 19-34, 19-35). It is an exceptionally complex structure whose walls bear some 40 calcareous ossicles and 80 muscles. It is divided into a large, dorsal cardiac stomach (or anterior chamber) and a smaller, ventral pyloric stomach (or posterior chamber).
The cardiac stomach is the large balloon-like structure in the anterior thorax. It lies dorsal to the mouth to which it is connected by the short esophagus (Fig 19-34). Its walls contain the ossicles of the gastric mill, used for grinding food. The ossicles are part of the exoskeleton and, being exoskeletal, are shed and replaced with each molt. The mandibles cut food into small pieces which are passed to the gastric mill for trituration and mixing with hydrolytic enzymes from the digestive ceca.
The pyloric stomach is the much smaller ventral region of the stomach. It lies posterior and ventral to the cardiac stomach and is hidden by it.
Look on either side of the mouth and esophagus to find the white calcareous internal part of the mandible extending into the anterior body cavity (Fig 9). The four powerful muscles that operate the mandible insert here, three of them by calcareous white tendons. Gently push the mandible back and forth and watch the response of the cutting edge. Two muscles, the lateral adductor and posterior adductor, move the cutting surfaces together (Fig 9, 11). Two other muscles, the abductor minor and abductor major, move the surfaces apart (Fig 9).
à Postpone examination of the remainder of the digestive system and then return to this part of the exercise AFTER study of the reproductive system. At that time, remove the reproductive organs in the vicinity of the heart if you have not already done so. GENTLY lift the posterior end of the cardiac stomach and look beneath it for the small pouchlike pyloric stomach (14, 19-35, 19-34). Do not damage the muscles and other tissues in this area. The walls of the pyloric stomach bear the abundant ossicles and fine setae of the filter press that is used to sieve particles from the liquid in the stomach. The liquid, which contains the products of hydrolysis, is sent to the digestive ceca for absorption.
While the proventriculus is elevated, find the delicate, transparent midgut running posteriorly from the posterior chamber under the heart to join the hindgut in the anterior abdomen (Fig 11).
The hindgut, or intestine, runs along the ventral midline of the abdomen to empty through the anus located on the ventral surface of the telson (Fig 2).
" With your scissors, make a transverse cut across the top of the stomach and look inside. Locate the ossicles of the gastric mill, find the pyloric stomach on the floor of the posterior half of the cardiac stomach. Find the filter press in the pyloric stomach. Insert the tip of the probe into the mouth and use it to locate the opening of the esophagus into the stomach.
Respiratory System
The respiratory system consists of the gills located in the two lateral branchial chambers (Figs 1, 11, 19-36). These large chambers occupy the pointed lateral sides of the body and are bounded on all surfaces by the skeletal branchiostegite of the carapace. The branchiostegite is a double fold of body wall enclosing the branchial chamber. It is the lateral carapace. Of its two layers, the outer is heavy and calcified and part of it has been removed. The inner layer is thin, uncalcified and unsclerotized and is still intact, covering the gills. This transparent body wall lying over the gills is no more than a thin chitinous membrane investing the dorsal surface of the branchial chamber. It is almost invisible but it is all that separates the branchial chamber (which is filled with seawater) from the hemocoel (which is filled with blood). Find and remove this thin, transparent sheet from the surface of the gills. The branchial chamber is now open and the gills are exposed for study.
There are eight gills on each side of the body but two of them are small and easily overlooked. The gills are exites of thoracopods. Each of the eight gills consists of a long central axisto which are attached, on opposite sides, two rows of very closely spaced flat branchial lamellae (Fig 19-37E,F). Use a microneedle to separate two adjacent lamellae from each other and look at them with magnification. Together the branchial lamellae provide an immense surface area for gas exchange. The lamellae are covered by cuticle which is molted periodically along with the rest of the exoskeleton.
" Snip the end from one of the gills, place it in a small dish (6-cm culture dish) of water and examine it with the dissecting microscope.
Most crabs and shrimps, including Callinectes, have lamellar (= phyllobranchiate) gills in which the respiratory surface consists of numerous flat lamellae radiating from a central axis (Fig 19-37E,F). Look at the cut surface of the gill axis to find the blood channels, cut in cross-section, that extend the length of the gill axis (Fig 19-37E). These include the afferent channel that delivers unoxygenated blood to the gill and the efferent vessel that drains oxygenated blood away from the gill.
The gills projecting into the branchial chamber divide it into dorsal and ventral regions (Fig 19-36). Water flows in the inhalant aperture to the ventral inhalant chamber (= hypobranchial chamber), then across the gill filaments into the dorsal exhalant chamber (= epibranchial chamber). It then exits via the exhalant aperture.
Insert a blunt probe into the inhalant aperture at the base of the cheliped and observe that it enters the ventral inhalant chamber, below the gills. The probe can be pushed gently upward through the curtain of gills into the dorsal exhalant chamber above the gills thus tracing the route taken by the respiratory water current through the gill chamber (Fig 19-38). Now insert your probe into the exhalant aperture and note that it enters dorsally, above the gills, in the exhalant chamber.
Water normally flows into the crab on its ventral side, up through the gills and then out dorsally (Fig 19-38). The water flow is generated by rhythmic undulations of the gill bailer. Reversing the beat of the bailer reverses the direction of flow over the gills. This backflushes the gills to clean them or to respire at the surface of poorly oxygenated water. Crabs attempting to respire out of water or in very shallow water may blow bubbles from the exhalent apertures beside the mouth if they do not reverse the direction of flow. Some crabs, such as the portunid lady crab,Ovalipes, burrow backwards into sand and accordingly employ reversed water flow through the branchial chamber. This avoids fouling the intake with sand and also allows the crab to utilize the posterior outflow of water to loosen the sand ahead of digging.
>1c. If living, unrelaxed specimens are available, place one in an 8" finger bowl of clean sea water. Tap water can be used if seawater is unavailable. The fingers of the chelipeds must be bound with elastic bands so the crab cannot pinch. Fill a plastic pipet with 1% toluidine blue/seawater solution or other non-toxic dye. Remove the crab from the dish. Insert the tip of the pipet into the inhalant aperture at the base of the cheliped and deliver 2-3 ml of dye deep into the branchial chamber. Place the crab back into the dish of water, watching its mouth field as you do. The flow of water through the branchial chamber will sweep the dye out of the chamber via the exhalent aperture. Watch the flow of colored water from the animal and determine the point at which it leaves the body. Does this correspond with the position of the exhalant apertures? Does the dye emerge from both exhalent apertures or only one? <
Lift the gills of your dissected specimen and look at the floor of the branchial chamber. Here you will see the narrow, setose flabella of the second and third maxillipeds in the inhalant chamber (Fig 11, 19-36). The flabellum of the third maxilliped is the longer of the two. With forceps wiggle the two maxillipeds in turn and watch their flabella move. These flabella are moved by the activity of their respective maxillipeds.
Look in the exhalant chamber along the anterior edge of the base of the gills to find the flabellum of the first maxilliped. This flabellum has its own muscles and operates independently of its maxilliped. Wiggle the first maxilliped and see that its flabellum does not respond as did those of the other two maxillipeds. The flabella sweep over the surface of the gills and keep them clean. The second and third maxillipedal flabella clean the inhalant side of the gills whereas the first maxillipedal flabellum cleans the exhalant side.
The flabella also participate in circulating water through the branchial chamber. The gill bailer of maxilla 2 can be seen beside the flabellum of maxilliped 1. The gill bailer beats to move water out the exhalant apertures and has the chief responsibility for generating the respiratory current through the branchial chamber.
In spite of the activity of the flabella the gills are sometimes fouled. The tiny stalked barnacle, Octolasmis (2mm) lives attached to the gill lamella of several species of crabs. In this position these filter feeders take advantage of a protected habitat in the branchial chamber through which there is a constant flow of food and oxygen rich water. You may see some of these on your crab’s gills. A small, orange, nemertean worm, Carcinonemertes carcinophila, also inhabits the branchial chamber of blue crabs.
Reproductive System
The morphology and appearance of the reproductive system vary markedly depending on sex and degree of maturity of the specimen. The following accounts are of mature individuals and if your specimen is immature you may not find all of the structures mentioned.
Male
The two long, paired, indistinct, white or grayish, testes lie dorsally in the anterior body where they may be difficult to distinguish from the digestive ceca beneath them (Fig 11). Each testis is a translucent convoluted tube that begins laterally near the base of the lateral spine and extends anteriorly and medially. It lies on top of the digestive ceca and parallels the anterolateral border of the carapace. Near the stomach it turns posteriorly and parallels the border of the stomach and approaches the midline.
Near the midline the testis becomes the complex, coiled and looped vas deferens of many regions and colors (white, pink, greenish). In Callinectes, the proximal vas deferens exits the testis and is located near the midline posterior to the stomach. It is a highly convoluted, small-diameter tubule wound on itself to form a globular mass. The white color is due to white spermatophores which are formed here of sperm from the testes. The spermatophores look like tiny white eggs.
The proximal vas deferens connects with the large, conspicuous, pink middle vas deferens which lies beside the lateral border of the stomach ventral to the testis. In mature specimens this is the easiest part of the male system to see. It is large, pink, and glandular in mature individuals. A pink jelly plug that will be transferred to the female during copulation is formed here.
The middle vas deferens turns ventrally at its anterolateral end and doubles back underneath itself to become the distal vas deferens. This large, thick, greenish, translucent, convoluted tube is not obvious, in spite of its size, because it is ventral to the proximal and middle vasa deferentia and is obscured by them. Part of the digestive ceca surround the vas deferens and hide them. You must remove this part of the ceca if you wish to see the distal vas deferens. The distal vas deferens makes several loops ventral to and posterior to the middle vas deferens and then narrows dramatically, ceases its coiling, and becomes a slender tube extending obliquely to enter the penis at the base of the last leg.
>1e. Remove a few spermatophores from the proximal vas deferens and place them on a slide with seawater or isotonic saline but do not add a coverslip yet. Look at the spermatophores with the dissecting microscope. They are ovoid in shape and are packed with spermatozoa. Affix a coverslip and withdraw enough of the water from beneath it so that some of the spermatophores rupture from the pressure of the coverslip. Examine the contents of a ruptured spermatophore with high power of the compound microscope (400X). The spermatozoa are small, irregular, unflagellated cells with short cytoplasmic processes. <
Female
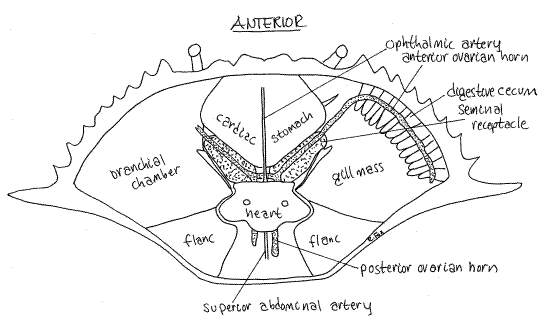
In the mature female, the orange ovaries may be small or so large they obscure other organs in the hemocoel (Fig 12, 19-35). In immature individuals, the ovary is beige or white and much less conspicuous. The right and left ovaries are connected across the midline by a narrow isthmus and each consists of anterior and posterior horns. Together the ovaries and the connecting isthmus form an "H" (Fig 12).
The oviducts exit the ovary and connect with the female gonopores on the sternite of thoracomere 6. The distal region of the oviduct is the seminal receptacle, part of which has a hard chitinous wall (Fig 12). Each of the two receptacles is located a little lateral to the midline between the stomach and heart along the posterolateral border of the stomach next to the gill mass. In the male the middle vas deferens occupies the equivalent position.
The size and color of the receptacle varies and may be quite large, hard, and pink for a brief period following copulation when the sperm mass and its large, pink, jelly plug are present. Later the receptacles shrink again and turn white as the jelly is absorbed. Sperm are retained in the seminal receptacle where they will later be used to fertilize the eggs. Copulation occurs while the ovary is still white and immature. Later it will turn orange and expand greatly in size as orange yolk accumulates.
Reproduction
Female blue crabs mate once and usually spawn twice, using the sperm from the single mating for both spawns. Female crabs mate following their terminal molt and the male crab carries his mate about awaiting this event. After the female molts, the male turns her on her back and inserts his gonopods into the female gonopores (Fig 19-47B). He deposits sperm in her seminal receptacles, which lie just inside the gonopore. Sperm remain here while the female migrates downstream in the estuary. Eggs are fertilized as they move through the oviduct to the exterior. Almost two million of them may be shed and attached to the setae of the pleopods and there begin their development.
Figure 12. Dorsal dissection of a fertilized but sexually undeveloped female crab. The seminal receptacle is filled with sperm but the ovary is small and undeveloped. Notice the "H" shape of the gonad. Crab31La.gif
Excretory System
The excretory system consists of two soft, grayish or pale greenish-white antennal glands located inside the anterior wall of the cephalothorax behind the second antenna (Fig 11). They may be difficult to find. The glands are saccate nephridia with an end sac derived from the coelom and a tubule and labyrinth derived from its coelomoduct (Fig 19-6B). Ultrafiltration of the blood occurs across the walls of the end sac and the filtrate is modified by the labyrinth. The final urine is stored in two very large, thin-walled, transparent bladders which empty to the exterior via the nephridiopores on the peduncle of antenna 1. The walls of the bladder are very thin and are not easily recognized, even when intact and undamaged.
These antennal glands are effective osmoregulatory organs and blue crabs are tolerant of a wide range of salinities. The glands play little or no role in nitrogen excretion, most of which occurs across the surfaces of the gills.
Nervous System
The nervous system is transparent and difficult to visualize in fresh material. It is slightly easier to see if a pipet of 99% isopropanol or ethanol is applied to the area inside the head and thorax. After a few minutes the nervous system will become opaque and white.
Figure 13. The somatic nervous system of the cancrid crab, Cancer. Redrawn from Pearson, 1908. crab50.gif
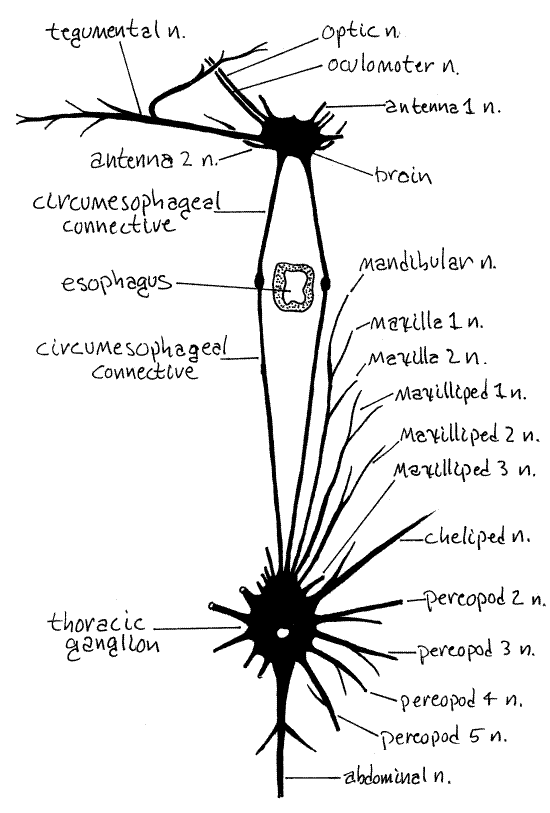
The nervous system of brachyuran crabs is highly cephalized into a brain and a large thoracic ganglion (Fig 13). The brain is located dorsally in the head immediately posterior to the rostrum, between the two eyestalks, and on the midline. It lies under a layer of muscle and connective tissue that must be removed before it can be seen. Emerging from the brain are optic nerves to the compound eye, oculomotor nerves to the eyestalk muscles, antenna 1 and antenna 2 nerves to the two pairs of antennae, and a tegumentary nerve to the anterior integument (Fig 13).
Two long circumesophageal connectives leave the brain to run posteriorly and ventrally around the sides of the esophagus. Well posterior to the esophagus they join the thoracic ganglion which is formed of the coalesced paired ganglia of all thoracic and abdominal segments. Paired nerves radiate from this ganglion to each thoracic appendage and a single abdominal nerve extends to the reduced abdomen and its appendages (Fig 13). The thoracic ganglion is obscured by connective tissue and muscle which must be removed to reveal it. It lies at the intersection of the midline and a line connecting the seventh anterolateral teeth of the two sides of the carapace (Fig 1). Segmental nerves to the thoracic segments exit this ganglion as does a single, median nerve to the abdomen.
Notice the large, movable transverse apodeme extending from one eyestalk to the other. It is a rigid connection between the two that insures the two eyes will act in unison. Move the apodeme and observe the response of the two eyestalks.
References
Bliss DE . 1982. Shrimps, Lobsters, and Crabs. New Century, Piscatawaty, New Jersey. 242p.
Bliss DE, Mantel LH(eds.). 1983. The Biology of Crustacea, vol. 5: Internal Anatomy and Physiological Regulation . Academic Press, New York. 471p.
Brooks WK . 1890. Handbook of Invertebrate Zoology. Bradlee Whidden, Boston. 352p.
Brown FA . (ed) 1950. Selected Invertebrate Types. Wiley, New York. 597p.
Cochran DM. 1935. The skeletal musculature of the blue crab, Callinectes sapidus Rathbun. Smithson. Misc. Coll 92(9):1-76.
Cronin LE. 1947. Anatomy and histology of the male reproductive system of Callinectes sapidus Rathbun. J. Morphology 81:209-239.
Crothers JH . 1967. The biology of the shore crab, Carcinus maenas (L.). Field Studies 2:407-434.
Harrison FW, Humes AG (eds.). 1992 . Microscopic Anatomy of Invertebrates vol. 10 Decapod Crustacea. Wiley-Liss, New York. 459p.
Ho J-S . 1978. Laboratory Manual for Invertebrate Zoology Emphasizing Marine Forms. Hwong, Los Alamitos, Calif. 152p.
Johnson PT. 1980. Histology of the Blue Crab, Callinectes sapidus. A Model for the Decapoda. Praeger, New York. 440p.
Kretz J, Bucherl W. 1940. Contribuicao ao estudo da anatomia e fisiologia do genero Callinectes (Crustacea Decapoda, Fam. Portunidae). Arq. Zool. Sao Paulo, 1:153-217.
Pearson J. 1908. Cancer. Liverpool Marine Biology Committee Mem. 16:1-217, 13 pls.
Pyle R, Cronin E . 1950. The general anatomy of the blue crab, Callinectes sapidus Rathbun. Chesapeake Biological Lab. Pub. 87:1-38.
Ruppert EE, Fox RS. 1988. Seashore animals of the Southeast. Univ. South Carolina Press, Columbia. 429 pp.
Ruppert EE, Fox RS, Barnes RB. 2004. Invertebrate Zoology, A functional evolutionary approach, 7 th ed. Brooks Cole Thomson, Belmont CA. 963 pp.
Smith RI . 1978. The midgut caeca and the limits of the hindgut of the Brachyura: A clarification. Crustaceana 35:195-204.
Warner GF. 1978. The Biology of Crabs. Van Nostrand Reinhold, New York. 202p.
Warner WW. 1976. Beautiful Swimmers. Penguin Books, New York. 304pp.
Williams AB. 1978. The swimming crabs of the genus Callinectes (Decapoda: Portunidae). Fish. Bull. 72:685-798.
Williams AB. 1974. Shrimps, Lobsters, and Crabs of the Atlantic Coast of the Eastern United States, Maine to Florida. Smithsonian Institution Press, Washington. 550pp.
Supplies
Dissecting microscope
Compound microscope
Slides and coverslips
Living or preserved blue crab
Large dissecting pan (about 20 cm long)
99 % isopropyl alcohol
Dissecting set
Rubber rings made by cutting cross sections of surgical rubber tubing
Callinectes Ringer's Solution: 2.444g NaCl, 0.086g KCl, 0.142g CaCl 2, 0.166g MgCl 2•6H 2O per 100 ml water at pH 7.3-7.5 (Pyle & Cronin, 1950).
Living Maryland blue crabs can be ordered from Chesapeake Crab Connection, www.ordercrabs.com . Sizes are 5–7+ inches with prices, per dozen, varying from $22-$75 depending on size. Shipping and handling vary with quantity and distance but will usually be at least $50. The web site will calculate the cost for you.