Invertebrate Anatomy OnLine
Bellamya japonica ©
Japanese Mystery Snail
24may2007
Copyright 2005 by
Richard Fox
Lander University
Preface
This is an exercise from Invertebrate Anatomy OnLine , an Internet laboratory manual for courses in Invertebrate Zoology. Additional exercises can be accessed by clicking on the links to the left. A glossary and chapters on supplies and laboratory techniques are also available. Terminology and phylogeny used in these exercises correspond to usage in the Invertebrate Zoology textbook by Ruppert, Fox, and Barnes (2004). Hyphenated figure callouts refer to figures in the textbook. Callouts that are not hyphenated refer to figures embedded in the exercise. The glossary includes terms from this textbook as well as the laboratory exercises.
Systematics
Mollusca P, Eumollusca, Conchifera, Ganglionura, Rhacopoda, Gastropoda C, Prosobranchia sC, Caenogastropoda O, Mesogastropoda sO, Ampullarioidea SF, Viviparidae F (Fig 12-125)
Mollusca P
Mollusca, the second largest metazoan taxon, consists of Aplacophora, Polyplacophora, Monoplacophora, Gastropoda, Cephalopoda, Bivalvia, and Scaphopoda. The typical mollusc has a calcareous shell, muscular foot, head with mouth and sense organs, and a visceral mass containing most of the gut, the heart, gonads, and kidney. Dorsally the body wall is the mantle and a fold of this body wall forms and encloses that all important molluscan chamber, the mantle cavity. The mantle cavity is filled with water or air and in it are located the gill(s), anus, nephridiopore(s) and gonopore(s). The coelom is reduced to small spaces including the pericardial cavity containing the heart and the gonocoel containing the gonad.
The well-developed hemal system consists of the heart and vessels leading to a spacious hemocoel in which most of the viscera are located. The kidneys are large metanephridia. The central nervous system is cephalized and tetraneurous. There is a tendency to concentrate ganglia in or near the circumenteric nerve ring from which arise four major longitudinal nerve cords.
Molluscs may be either gonochoric or hermaphroditic. Spiral cleavage produces a veliger larva in many taxa unless it is suppressed in favor of direct development or another larva. Molluscs arose in the sea and most remain there but molluscs have also colonized freshwater and terrestrial habitats.
Eumollusca
Eumollusca, the sister taxon of Aplacophora, includes all molluscs other than aplacophorans. The eumolluscan gut has digestive ceca which are lacking in aplacophorans, the gut is coiled, and a complex radular musculature is present.
Conchifera
Conchifera, the sister taxon of Polyplacophora, includes all Recent molluscs other than aplacophorans and chitons. The conchiferan shell consists of an outer proteinaceous periostracum underlain by calcareous layers and is a single piece (although in some it may appear to be divided into two valves). The mantle margins are divided into three folds.
Ganglioneura
Most Recent molluscs are ganglioneurans, only the small taxa Aplacophora, Polyplacophora, and Monoplacophora are excluded. Neuron cell bodies are localized in ganglia.
Rhacopoda
The mantle cavity is posterior in the ancestor although it may be secondarily moved to an anterior position by torsion. This taxon includes gastropods and cephalopods.
Gastropoda C
Gastropoda is the largest molluscan taxon and is the sister group of Cephalopoda. Gastropods are united by descent from a torted ancestor although many exhibit various degrees of detorsion. Many are coiled and asymmetrical but the ancestor was probably symmetrical. Gastropods are relatively unspecialized molluscs known collectively as snails. The univalve shell, present in the ancestral gastropod and in the majority of Recent species, is reduced or lost in many representatives. The flat creeping foot was inherited from their eumolluscan ancestors but gastropods have developed a distinct head with an abundance of sophisticated sense organs. The originally posterior mantle cavity has become anterior as a consequence of torsion, although detorsion has reversed this condition in many. Gastropods were originally gonochoric and most remain so but many derived taxa are hermaphroditic. Most are marine but many taxa have invaded freshwater and the only terrestrial molluscs are gastropods. Most have a single gill, atrium, and nephridium but the most primitive representatives have two of each. Only one gonad, the right, is present. The ancestor probably had an operculum. The nervous system is streptoneurous (twisted by torsion).
Prosobranchia sC
Prosobranchia was once one of three great gastropod subclasses but it is no longer considered to be a monophyletic taxon, although the concept continues to be used as a pedagogical convenience. Prosobranchs are the gastropods most like the ancestral snails. They are torted and most have a shell and are coiled and asymmetrical. The mantle cavity is anterior. Most are gonochoric and most have an operculum. Most have only one gill in the mantle cavity but some primitive taxa have two. The right atrium is lost in most. Prosobranchs are specialized for life in marine benthic habitats although representatives are also found in freshwater and on land.
Caenogastropoda O
Caenogastropoda includes the two large and successful groups, mesogastropods and Neogastropoda. One gill, one nephridium, and one atrium are present. The gill is monopectinate, with filaments on only one side of the central axis. This new gill is less prone to fouling with sediment and silt and is probably largely responsible for the success of these snails as it allowed invasion of soft-bottom habitats.
Mesogastropoda sO
Although Mesogastropoda is no longer thought to be monophyletic it remains a useful pedagogic device and the concept is still widely employed. The mesogastropod osphradium is a simple ridge and the radula is taenioglossate, with seven teeth in each transverse row. Mesogastropods occur in marine, freshwater, and terrestrial habitats but most are marine.
Viviparidae F
These snails are viviparous and females are equipped with a uterus in which they gestate the eggs until they become juvenile snails. They release fully developed small snails, hence the scientific name Viviparidae and the common name "live-bearing snail".
Viviparids are large snails resembling their relatives the apple snails (Ampullariidae = Pilidae) but, in contrast with ampullariids, viviparids are not amphibious, do not have a lung, do not respire in air, and do not lay eggs. The male viviparid right tentacle is modified to serve as a penis. Ampullariids, which, like viviparids, are freshwater prosobranchs, have a lung and a gill, are amphibious and can respire in either air or water, lay calcareous eggs above the water line, are oviparous and do not gestate, and have identical right and left tentacles in both sexes. Mystery snails tend to have higher spires than apple snails.
Natural History
Viviparids are large freshwater snails known as “mystery snails” or “live bearing snails”. Mystery snails found in North America belong to several genera (Campeloma, Viviparus [ = Paludina] , Bellamya [ = Cipangopaludina] , Tulotoma, and Lioplax) in the family Viviparidae. Five genera and 19 species of viviparids occur in North American habitats. Of these most are native but some are introduced exotics. Bellamya is introduced, but Campeloma, Lioplax, and Tulotoma are endemic to North America. Viviparus is not endemic but is widespread and common in eastern North America where it is found in both lakes and rivers. Tulotoma is restricted to the Coosa River of Alabama.
Most viviparids are detritivores or microphagous browsers feeding on microalgae. Bellamya is a filter feeder and detritivore, but also browses on microalgae. In aquaria, and presumably in nature as well, Bellamya eats carrion when available. It does not feed on macrophytes, making it popular with aquarists and water gardeners. The spread of these snails across North America has probably been facilitated by these hobbyists. It sometimes appears in North American food markets catering to Asians. That a single gravid female is capable of founding a population helps explain their rapid dispersal.
With few exceptions viviparids are gonochoric. Females retain and gestate eggs in an enlarged uterine region of the pallial oviduct. Large shelled juveniles are released by the female at short intervals. Sexual dimorphism in Bellamya is slight, with males being smaller than females and with their right tentacle modified as an intromittent organ (penis).
Laboratory Specimens
Because of the effects of torsion and coiling on the molluscan body plan, gastropod anatomy can be confusing to beginning students and achieving an understanding of it is an objective that should be served by the laboratory experience. Unfortunately, the preserved snails traditionally used for this purpose are difficult and frustrating to dissect and often fail to improve the student's grasp of gastropod anatomy. Relaxed recently killed or living anesthetized snails, which have not been preserved, are much easier and more rewarding to dissect and are more likely to further an understanding of molluscan anatomy and the effects of torsion and coiling.
Viviparids are good representative mesogastropods, and of prosobranchs in general. Their value in the study of invertebrate zoology is enhanced by the fact that living specimens are often be available inexpensively and that most anatomical features can be observed with minimal dissection. For zoology courses taught at inland locations, viviparids are a convenient alternative to marine snails such as Littorina or Buccinum. Sometimes large specimens of various viviparid (or ampullariid, see the link to Pomacea) species can be purchased at reasonable cost from pet or garden stores. This trade has been curtailed somewhat by legislation prohibiting the sale of some species of freshwater snails because of the tendency for escaped individuals to found permanent invasive populations (of which Bellamya is an excellent example). Viviparids are usually called “mystery snails” or “live bearing snails” in the pet trade but the names and identifications used in many shops are not reliable. Ampullariids include the apple, ram’s horn, golden, and blue snails of the pet trade. This exercise should not be used with ampullariid snails. Use the Pomacea exercise instead.
Freshwater habitats in North America support several species of viviparid snails that can be used for the study of gastropod anatomy. The ideal choice would be one of the two large introduced viviparids, Bellamya japonica and B. chinensis, that are widespread and in some localities may be available in large numbers. They are introductions that should not be in North American habitats in the first place. Further they are not endangered or protected.
This exercise is written specifically for the introduced snail, Bellamya japonica (= Cipangopaludina japonica, the Japanese mystery snail or giant Asian snail), which differs only slightly from its congener Bellamya chinensis (= Cipangopaludina chinensis, = Viviparus malleata, the oriental mystery snail), also an exotic, naturalized species. The two are similar and some specialists consider them to be conspecific. Any other suitably large viviparid could also be used but most of these are natives.
The two Bellamya species were introduced around 1890 and are now widespread and apparently still spreading. They are harvested for food in Asia and also in North America to serve Asian markets. Bellamya achieves much larger sizes, up to 70 mm in shell height, than any of our native viviparids, of which the maximum is about 35 mm. Bellamya japonica is more high spired and angular than the similar B. chinensis but both species exhibit variability in shell shape.
Many anatomical features of Bellamya are visible on the surface of the animal (after removal from the shell, of course) and require little or no dissection. One great advantage is that almost all of the gut is visible on the surface making it relatively easy to trace its course through the body (a tedious and often unsuccessful task in most gastropods). When dissection becomes necessary it is usually minimal.
This exercise was prepared using specimens collected from the Saluda River immediately below the Buzzard’s Roost dam, which impounds the waters of Lake Greenwood in the South Carolina Piedmont. Bellamya was first reported from Lake Greenwood in 2005 by which time it was already abundant. Specimens 70 mm in shell height were been collected from Lake Greenwood during the preparation of this exercise.
Active Snails
> a. Place a living unanaesthetized Bellamya in a 12-cm culture dish of lake water on the stage of a dissecting microscope. If you take the time to do this you will see some features of the snail under natural, active conditions, which is more instructive, and more fun, than seeing them after being anesthetized or sacrificed.
Arrange the snail in the dish so its aperture (opening) and operculum are up, facing you, and then leave it completely alone without bumping, jarring, sliding or otherwise disturbing it. The slightest disturbance, including a shadow, will cause it to retract. Focus on the left side of the snail. At first the animal will be retracted completely within the shell whose opening, the aperture, will be plugged by a black, teardrop-shaped door, the operculum. While you are waiting you might look for epifaunal organisms growing on the outside of the shell.
When it feels safe, the snail will move the operculum away from the aperture and extend its foot and head. You will see the dark slate gray integument, speckled with gold pigment spots. The gold color fades after death.
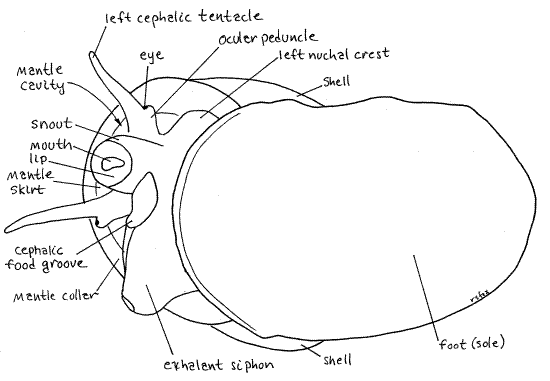
A snail body consists of foot, head, and visceral mass. Of these, the head and foot are visible but the visceral mass is completely enclosed in the shell which it never leaves. At first the large muscular foot will be folded on itself so you cannot see its true shape. The operculum is attached to the foot. The small head sits atop (dorsal to) the foot at the anterior end. You will probably be looking at its left side and if so you will see the left cephalic tentacle with a small eye near its base. The eye is on a short ocular peduncle fused with the lateral edge of the tentacle. To the right of the left tentacle, and thus on the midline of the head, is the short tubular snout with the mouth in the center of its free distal tip. You will not see the mouth unless the animal happens to point the snout at you while you are watching. A right tentacle is present to the right of the snout but you probably won’t see it if you are looking at the left side. Don’t try to reposition the snail or it will retract into the shell.
Above the head you will see the large arch formed by the edge of the shell. Lining this arch is a ridge of tissue known as the mantle collar. It is the edge of the mantle skirt. The edge of the shell arches over a large opening into the spacious cavelike mantle cavity. The mantle skirt is the roof of the mantle cavity. Focus through the opening on the numerous, fine, parallelfilaments of the gill hanging from the roof.
Look at the left side of the inconspicuous neck connecting the head with the foot. To the left of the left tentacle you will see a thin earlike flange of tissue, the left nuchal lobe (nuchal = neck). The snail can roll this lobe into a short tube and use it as an inhalant siphon to bring water into the mantle cavity to flow over the gill. A similar, but larger, right nuchal lobe (exhalant siphon) is present to the right of the right tentacle but you probably won’t see it from your viewpoint.
The snail will attempt to extend the foot and right itself with the sole of the foot down, against the bottom of the dish. If it accomplishes this, you will get to see the foot in its normal, extended condition with the operculum attached to the top of its posterior end. The anterior end is under the head. When the snail contracts it will fold the foot in half with the anterior and posterior ends of the sole facing each other and then withdraw it into the aperture. This leaves the operculum on the outside, blocking the aperture. <
> b. If active undisturbed snails are maintained in an aquarium in your laboratory, spend a little time observing them without disturbing them. In these conditions you can see the features observed earlier under more natural conditions. Viewing will be best if you can find a snail on the front wall of the aquarium so you can look through the glass to see the animal (Fig 1, 2).
Under these conditions the foot is seen fully extended and in motion. Its ventral surface is the sole, which you can see is a pale, broad, flat oval attached to the glass. The operculumis on the posterior dorsal surface of the foot and may be out of your line of sight. The head is dorsal to the anterior end of the foot. The large cylindrical snout protrudes anteriorly from the midline of the head. The slitlike mouth is in the center of the free, distal tip of the snout. You will probably see the snail apply the tip of the snout to the substratum as it feeds on microalgae growing on the glass. If the snail is on the front glass of the aquarium, you may be able to see the rasplike radula protrude from the mouth and scrape against the glass to remove algae.
Figure 1. Bellamya in ventral view on the glass wall of an aquarium. Gastrop77L.gif
A conspicuous cephalic tentacle can be seen on each side of the snout. In males the right tentacle is thicker than the left and is curved at the tip. It functions as a penis and its presence is the only reliable external means of determining the sex of an individual. Watch as the animal uses the tentacles to investigate the environment ahead of its motion. A small black eyeis situated laterally near the base of each tentacle at the tip of an ocular peduncle. The head connects with the foot and visceral mass via a short neck.
If your viewing angle permits, you may see, above the head, the opening into the mantle cavity. The shell, of course, encloses the visceral mass and is dorsal to the foot and posterior to the head (Fig 4).
An inhalant siphon is present on the left side of the neck and an exhalant siphon on the right. Both are formed when the animal rolls the left and right nuchal lobes, into tubes. Theexhalant siphon, on the right, is much larger and usually the easier to see (Fig 1). In an undisturbed active animal it is a large diameter tube on the right of the snout. Be sure you understand how the snail can make a tube out of a flat nuchal lobe. You could do the same thing with a piece of paper. The inhalant siphon is to the left of the snout. A short ridge of tissue encloses thecephalic food groove and can be seen extending from the base of the right tentacle to a position under the snout (Fig 1). The groove transfers food from a food collection device in the mantle cavity to the mouth. <
Figure 2. A male Bellamya in ventral view from the left as it crawls on the glass wall of an aquarium. Gastrop83L.gif
> c. If adult Bellamya have been maintained in a laboratory aquarium for a few days, juvenile snails may be present (Fig 3). If so, place one of the small snails in an 8-cm culture dish of lake water and observe it with the dissecting microscope. Juveniles are not as shy as adults and you won’t have to wait long before it emerges from its shell. Start out with the snail upside down with its aperture facing up, toward you. Watch as the snail extends its foot and head from theshell. Note the sequence of events involving the operculum, foot, and head. It is the reverse of the sequence employed when the snail withdraws.
At first the animal is completely withdrawn with the operculum covering the aperture. The operculum moves aside as the folded foot, with the operculum stuck to the dorsal side of its posterior half, emerges first. Then the head emerges and the foot unfolds and attaches to the substratum. As the head emerges you will get a quick look at the snout, left cephalic tentacle, left nuchal lobe, mantle, and mantle cavity but as soon as the anterior foot contacts the substratum it will pull the shell around onto its life position above the foot so you can no longer see much of the head. Turn the snail back over so it has to emerge again to give you another look. Repeat this as often as necessary to see everything you want to see. Try to get a look into the mantle cavity to see the gill on its roof.
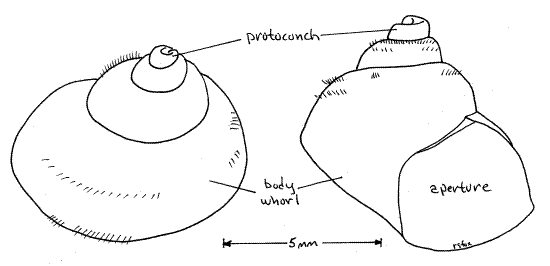
Look at the shell. It is fragile, compared with that of adults, and consists of a large diameter body whorl at its base with progressively small and smaller whorls spiraling above it (Fig 4). It probably has four whorls, or turns around its central axis. In these young snails the protoconch is still present and undamaged. The protoconch is the oldest and smallest whorl of the shell and sits at the apex. It is the shell of the larval or late embryo snail and because of its delicacy it is rarely present in adult snails.
The long, dark, tubular rectum may be visible through the translucent shell. The rectum is a long spiral band just under the shell of the body whorl. It is usually darker than the surrounding tissues. <
Figure 3. Two views of a 7 mm juvenile snail removed from the uterus of a 55 mm female. Gastrop92L.gif
Relaxation and Anesthetization
Fresh specimens for dissection should be relaxed/anesthetized so that the foot and head are extended from the aperture and then extracted from the shell. The specimen may be dead or alive and anesthetized. Molluscs are notoriously difficult to relax in an extended, relaxed condition. Under almost any adverse conditions, including immersion in anesthetic, the snail withdraws into its shell and waits for conditions to improve. It is possible to dissect unrelaxed snails but relaxed specimens are better subjects. Unrelaxed snails will have the foot folded tightly on itself and the head will be retracted into the mantle cavity. Relaxed specimens usually are only partly relaxed and the foot will be loosely folded. Four methods for preparing snails are suggested below but none is ideal. Instructions for extracting the snail from its shell appear later, in the Extraction section.
Method 1. Kill the snail by freezing it slowly in a 12-cm culture dish of tapwater. The snail must not experience any disturbance while freezing. The snail will usually freeze in a partially extended, dissectible condition. Freezing requires several hours and should be initiated by the teaching staff the day prior to the lab. Each snail should be frozen in a separate container of water so that the snails do not disturb each other. The snails should be placed upside down, with the aperture up, in their dishes. This technique results in partially relaxed, dead specimens but may present logistic difficulties for the teaching staff.
Method 2. Remove an unrelaxed snail from its shell (see Extraction section below). The snail can then be studied unrelaxed and unanesthetized or it can be immersed in 7 % non-denatured ethanol (made up in lake water) for relaxation and anesthetization. Study of the snail can begin immediately and the snail will die after about four hours immersion. If ciliary currents are to be studied the snail should not be placed in alcohol. This method minimizes demands on the teaching staff.
Method 3. A technique that results in living partially relaxed specimens is to place the snail in a dish or dissecting pan of 7% non-denatured ethanol with the foot extended and the aperture open. The anesthetic must have access to soft tissues which means the animal cannot be allowed to close the aperture. Surprise an extended animal in the aquarium and, before it can withdraw, prop the operculum open with something, such as a piece of a wooden applicator stick placed crossways between the foot and shell so the operculum cannot close and the anesthetic can reach the soft tissues. Hold this bridle in place with rubber bands and immerse the snail in alcohol. This is also a lengthy process requiring several hours and should be initiated early by the teaching staff. It is labor intensive.
Method 4. A snail can be placed in a culture dish completely filled with boiled (and thus anoxic), then cooled, water and covered with another dish so the snail has no access to oxygen. The dish must remain undisturbed during the process. The snail will eventually succumb in a partially extended condition and can be transferred to 7 % ethanol. The snail will be alive and partly relaxed.
Anatomy
Shell
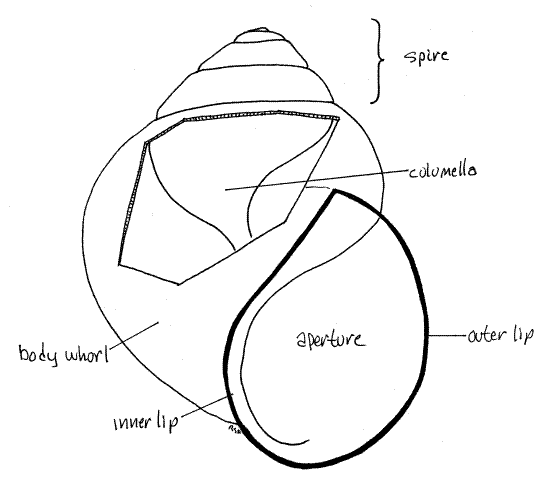
Examine the external features of an empty or occupied shell. The snail inhabits a shell composed of calcium salts and protein. (The thickness of the shell varies with species and environmental conditions.) The gastropod shell is a cone wrapped around a central axis to form a spiral consisting of a series of revolutions, or whorls, around the axis (Fig 4, 12-27A,B). The shell of Bellamya is inflated (with swollen sides). It consists of one large body whorl, which contains most of the soft parts of the animal, and several smaller whorls that form a spire extending above the body whorl.
The body whorl opens to the exterior via a large opening, the aperture, which is on the right side of the central axis of the shell when the spire is pointing up and the aperture is facing you. Snails with the aperture in this location are said to be dextral, or right-handed (Fig 4). If the aperture were on the left, the snail would be sinistral, or left-handed. Bellamya, like most snails, is dextral. The aperture of most viviparids is less than half the height of the shell.
The whorls are wound spirally around a central axis called the columella (Fig 4, 12-27A). This axis is in the center of the spiral of whorls and consequently is not visible in an intact shell. The shell is covered on the outside by a thin greenish brown, proteinaceous periostracum but the remainder of the shell is calcium carbonate deposited on a protein matrix. The periostracum, operculum, and matrix are made of the protein conchiolin. Under magnification, use a scalpel to scrape a small spot on the shell to remove the periostracum and reveal the underlying white calcareous portion of the shell.
The operculum is a thin teardrop-shaped disk of flexible conchiolin that forms a door to close the aperture. It is attached to the foot of the snail as you will soon see. In viviparids, the growth lines of the operculum form concentric rings around a "center", or nucleus.
Figure 4A. The shell of Bellamya. A window has been cut in the body whorl to reveal the columella. Gastrop175L.gif
4B. The operculum. Gastrop74L.gif
Extraction
The currently visible soft anatomy of your specimen (Fig 1, 2, 2-16C, D) includes the large, flat-soled foot which, if your specimen is incompletely relaxed, is probably partly folded on itself along a transverse crease. The head is dorsal to the anterior end of the foot. The visceral mass extends from the dorsum of the foot up into the whorls of the shell and is not visible in intact specimens. The snail must be removed from its shell before soft anatomy can be studied.
A snail is attached to its shell by a single muscle which must be detached in order to remove the animal without damage. This attachment is the large, white right columellar muscle which extends from the posterior dorsal area of the foot to the columella, or central axis, of the shell. Its origin on the columella cannot be accessed from the intact aperture so you will have to crack and remove most of the shell to get to it.
" Remove the snail from its shell as follows. Place the body whorl in a small C-clamp (or small vise) and crack the shell with pressure applied slowly. Do not attempt to do this with a hammer. The C-clamp permits controlled application of pressure that is not possible with a hammer. Once the shell cracks, immediately release the pressure and use heavy forceps to remove the broken pieces of shell. Reposition the remaining shell in the clamp and apply pressure again until something else breaks. Never apply pressure to anything that yields, either broken shell or soft tissue. Remove the broken pieces of shell as before.
Continue this process until you have removed most of the body whorl and can see the white columella in the core of the shell (Fig 4, 12-27A). Find the large white columellar muscleextending from the foot to the columella. The columellar muscle has a broad oval origin on the columella. Use a scalpel to scrape, not cut, the muscle away from the columella. (Freezing sometimes causes the muscle to detach from the columella spontaneously making it unnecessary to scrape it loose.) Now gently try to unwind the snail out of the remaining shell. Do not use force to remove the snail. If the snail does not slide out readily, resume cracking. Check for an intact part of the muscle attachment you missed. Do not pull on the snail to remove it. It should yield to a very gentle tug. More than that will tear the tissue of the visceral mass and make things difficult for you later.
Examine the extracted animal. Copious amounts of opaque, elastic, white mucus will be present and should be removed as it appears. Most of the mucus will be in the mantle cavity, on the head, or the sole of the foot, i.e. the surfaces exposed to the environment in a normal snail. Use a disposable plastic Pasteur pipet to wash the mucus away. You can also take the snail to a sink and run a gentle stream of water over the sole of the foot, head, and into the mantle cavity to rinse away the mucus. Whatever method you use, be gentle with your specimen. Without their shells molluscs are delicate and easily damaged. Continue to remove mucus as necessary throughout the dissection.
Soft Anatomy
Place the extracted snail in a small dissecting pan and cover it with water or7% ethanol. Place it on the stage of a dissecting microscope.
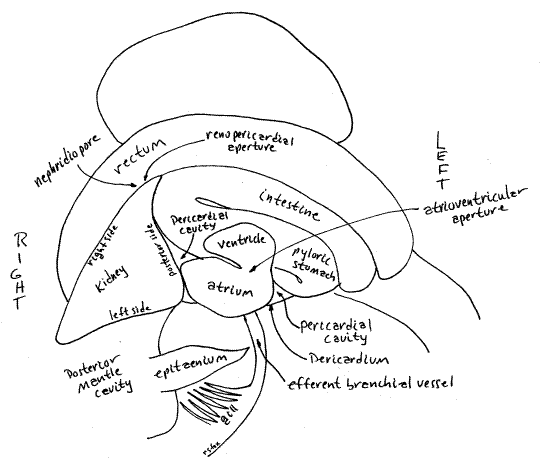
Make a preliminary survey of the major features of your extracted snail (Fig 5, 11, 12-14). The broad, normally flat, muscular foot is ventral to everything else but it is probably partly or completely folded so the anterior half faces the posterior half on either side of a transverse fold in the middle of the foot. The foot is tough and rubbery. The head, with two lateral tentacles and a median snout, is located above the anterior end of the foot. In unrelaxed specimens it will be wholly or partially withdrawn and hidden under the mantle. The integument of the head is variously pigmented in different species but that of Bellamya is black or lead gray with striking gold chromatophores (in life).
The remainder of the body is the visceral mass. It is coiled and sits on the dorsal side of the foot. All of the body posterior to the head and dorsal to the foot is visceral mass. It is tapered and spiraled to fit in the upper whorls of the shell. It contains most of the snail's organ systems, which in this species, are conveniently visible through the body wall (Fig 5, 18, 11). The most obvious of these organs are the dark tubular rectum, conspicuous on the surface of the second and body whorls, and the large yellowish-brown digestive ceca occupying, to the exclusion of everything else, the uppermost whorls and sharing the intermediate whorls with other systems. The body wall covering the visceral mass is thin and is usually translucent so organs under it can be seen without dissection. In some specimens it may be pigmented making it more difficult to see the internal organs. If so, the black pigment can usually be scraped or picked away to leave the body wall transparent.
Figure 5. Dorsal view of a male Bellamya with the shell removed. The animal is partly relaxed and the foot is folded on itself. Most of the head is retracted into the mantle cavity. Gastrop71L.gif
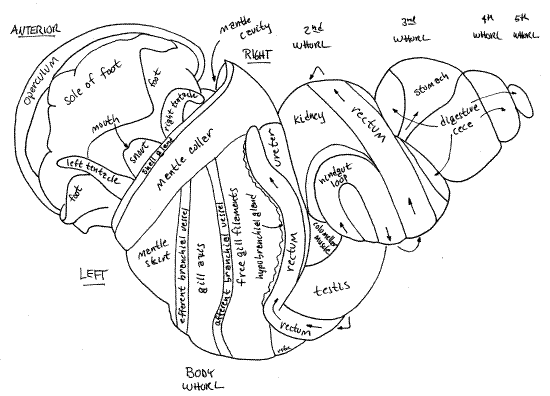
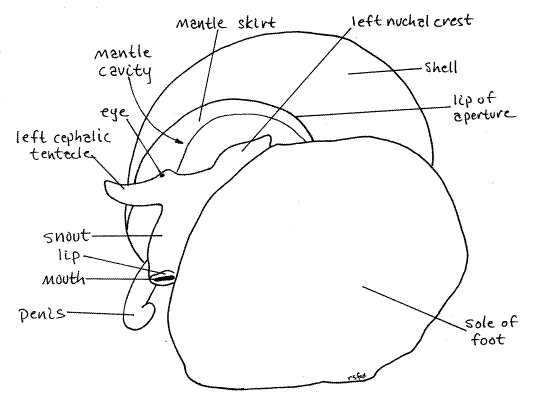
The mantle (= pallium) is the dorsal body wall of the visceral mass (Fig 1, 12-14A) invaginated to form a double layer of itself over a deep dorsal pocket. The large pocket thus formed is the mantle cavity, which in life is filled with water (Fig 12-53). It is an important feature of mollusc anatomy. The double sheet of mantle is the mantle skirt. The mantle skirt is the roof of the mantle cavity and is a double layer of the body wall. The floor of the mantle cavity is the non-doubled, non-folded dorsal body wall. In fresh specimens the mantle skirt is pale gray or white, in sharp contrast with the black and gold of the head and dorsal foot.
You are now ready for a more detailed study of gastropod anatomy.
Foot
The foot is a large mass of muscle making up the ventral part of the snail (Fig 1). Its smooth (when extended), oval, ventral surface is the sole and is the part that contacts the substratum when the snail is crawling. It is equipped with abundant mucous gland cells whose secretions lubricate the substratum over which the snail crawls. If you are working with a fresh specimen you have no doubt encountered the copious mucus secreted by these cells.
When retracted, the foot folds transversely across its middle to fit into the available space inside the body whorl of the shell. The foot of your specimen is likely to be folded in this manner (Fig 5). If it is, the anterior and posterior halves of the sole will be facing each other. The horny proteinaceous operculum is a thin brown disc attached to the dorsal surface of the posterior half of the foot (Fig 4B). It is a door to close the shell aperture when the foot and head are retracted.
Head
The head of your specimen may be withdrawn entirely or partially under the mantle skirt. If it is, push the skirt out of the way to expose it. It is connected to the anterior visceral mass by a short neck (Fig 12-14A). The head and foot are unaffected by torsion and coiling and exhibit bilateral symmetry. Find the right and left sides of the head and foot. The visceral mass, on the other hand, is asymmetric, being both torted and coiled.
The anterior end of the viviparid head bears a cylindrical median snout with the mouth at tip (Fig 5, 1). The mouth is flanked by a pair of fleshy lips (Fig 2, 13). The snout is not a retractable proboscis such as is found in some snails. Two long cephalic tentacles arise at the base of the snout, one on each side. The tentacles may be partially contracted and not as long and slender as they would be in life. Laterally, near its base, each tentacle bears a small but conspicuous black eye on a short ocular peduncle (Fig 1). The ocular peduncle is fused with the tentacle.
The right tentacle of male viviparids is modified to serve as a penis, or intromittent organ. Relaxed, it is thicker and is tip is slightly blunter than that of the left. The tip is held in a curve like a shepherd's hook. If you think your specimen is a male, examine the tip with higher magnification (about 20X) to find the tiny distal male gonopore. With fine forceps gently squeeze the penis while watching the tip. A thin stream of semen will be extruded from the opening (of mature specimens) to verify its presence.
Lateral to the tentacles, the two sides of the neck each bear a thin flap of tissue, called a nuchal lobe (nuch = neck) that can be rolled into an extensible tube, or siphon. Find the left andright nuchal lobes (= labial organs, nuchal crests, epipodia). When rolled, the left lobe forms the tubular inhalant siphon to bring a respiratory water current into the left side of the mantle cavity where it flows over the gill. The equivalent on the right is the exhalant siphon through which the respiratory current exits the mantle cavity. These lobes are highly extensible but in contracted specimens they are small. In dead or relaxed specimens the lobes will not be rolled to form tubes. The right lobe is much larger than the left and when rolled forms a larger, more noticeable tube. Between the right nuchal lobe and the snout is an additional low, thin ridge of tissue that forms a short tube. It curves around the outside of the base of the right tentacle and ends near the mouth. This is the cephalic food groove and it is a part of a filterfeeding apparatus which will be discussed later.
Mantle Cavity
A double fold of the mantle, known as the mantle skirt, arches over the anterior dorsal visceral mass. The deep, triangular pocket below the skirt is the mantle cavity (Fig 1, 5, 12-53). The mantle cavity covers the dorsal surface of the body whorl but extends, as the narrowing apex of an elongate triangle, around the entire revolution of the body whorl. The base of the triangle is anterior, above the head. Gently slip a blunt probe into the mantle cavity and plumb its depths.
The mantle skirt (= mantle fold, mantle flap) is the roof of the mantle cavity. The mantle cavity opens to the exterior via a large opening above the neck and under the skirt. The floor of the mantle cavity is the dorsal body wall of the anterior visceral mass.
The free anterior edge of the mantle skirt encircles the dorsal body as a opaque mantle collar, located immediately posterior to the head and surrounding the opening into the mantle cavity. It is thicker than the rest of the skirt and consequently is opaque. In an intact snail the mantle collar lines the free edge of the shell around the aperture, as you observed earlier (Fig 1).The mantle collar fits against the edge of the shell around the aperture and the epithelium of its anterior border secretes the growing edge of the shell. Growth of the shell is dependent on the secretory epithelium of the free mantle collar. This secretory anterior edge of the mantle collar is distinguishable as the shell gland (not to be confused with the female's egg shell gland).
Several organs are associated with the roof of the mantle cavity and many important features of snail anatomy can be seen here prior to dissection. The mantle cavity of viviparids, like that of most prosobranchs, is a single undivided space containing, among other organs, a gill. This contrasts with that of the related ampullariids in which the mantle cavity is divided into a gill chamber (= branchial chamber) for aquatic respiration and a lung for aerial respiration. The mantle cavity of pulmonates, such as Helix and Limax, is an air-filled lung and no gill is present.
Orient the snail in the dissecting pan with the foot and operculum down, against the wax and the dorsal surface of the head up. The anterior end should be toward you and the posterior end extending away from you. Anchor the snail in place by forcing two stout # 7 stainless steel insect pins through the operculum to hold it flat against the wax of the dissecting pan. Be prepared to remove these pins when necessary to reorient the snail.
Begin your study of the mantle cavity with an examination of the outer surface of its roof. With forceps stretch the roof taut so its surface is more or less flat. Scrape any black pigment off of the surface. Many features are visible externally as oblique bands extending right to left across the roof (Fig 5). The left side of the roof is the same in both sexes but the right side differs. The structures in the roof of the mantle cavity are, in order from left to right, the efferent branchial vessel (narrow, white, transparent tube), osphradium (short, narrow, white line but barely visible externally), gill axis (broad white band), afferent branchial vessel (narrow, yellowish, transparent tube), open area over the free ends of the gill filaments (wide, white, translucent area), hypobranchial gland (narrow, yellowish-white band), rectum (dark, conspicuous, band), and ureter (translucent inconspicuous band, and uterus (females only, very large, transparent, with small snails inside). In females the ureter is difficult to distinguish from the adjacent uterus. In males the ureter is easily seen and the tip of the testis can be seen to the right of the upper ureter (Fig 5, 18). Although visible over most of the mantle skirt, these organs cannot be seen through the thicker mantle collar along the anterior border of the skirt.
" Open the mantle cavity using fine scissors to make a shallow oblique cut across the roof of the cavity along the right margin of the gill axis. This cut should be very shallow to avoid cutting the gill filaments which extend from the gill axis to the right, towards the hypobranchial gland. Deflect the right and left sides of the roof and look at the inner surface of the left side.
The gill (= ctenidium) is on the left side of the roof of the mantle cavity (Fig 5, 12-14B). It consists of a wide longitudinal central axis from which numerous ciliated filaments extend to the right. The central axis is the band of tissue between the two branchial blood vessels. The filaments are unusually long and slender and attach to the mantle roof along the central axis from which their free ends extend far to the right in the mantle cavity. The filaments are not held together by tissue or ciliary interfilamentary junctions and easily become separated from each other and appear disorganized when disturbed.
> d. With fine scissors remove a few filaments by cutting across their bases. Transfer them to a slide and make a wetmount for examination with the compound microscope. A longitudinal muscle is easily seen extending the length of the gill. Estimate the ratio of filament length to the width of the base. That of Viviparus viviparus is 26:1) <
This is a monopectinate gill with only one row of filaments arising from the axis. All mesogastropods and neogastropods have monopectinate gills. The gill extends all the way to the posterior end of the mantle cavity and you cannot see all of it in dorsal view. The left gill is the only one present in almost all prosobranchs, including viviparids.
Afferent and efferent branchial blood vessels extend along the two long margins of the gill (Fig 5, 11, 12-52). The afferent vessel from the body (kidney) is on the right, the efferent to the heart is on the left side of the gill axis. These deliver unoxygenated blood to the gill (afferent vessel) and drain oxygenated (efferent vessel) blood from it. The efferent vessel empties into the atrium of the heart.
The narrow, sometimes inconspicuous, sensory osphradium is attached to the roof of the mantle cavity along the base of the inhalant (left) side of the gill and efferent branchial vessel. In most prosobranchs the osphradium is obvious and resembles a miniature bipectinate gill with a central, longitudinal axis from which arise two rows of filaments. The viviparid osphradium has no filaments and does not resemble the gill at all. That of Bellamya is a short yellowish-white line extending a few millimeters along the anterior end of the gill and efferent branchial vessel.
The respiratory current enters the mantle cavity trough the inhalant siphon (formed of the enrolled left nuchal lobe) on the left and crosses the osphradium. It then passes posteriorly in the left side of the mantle cavity and flows across and between the gill filaments to the right side of the mantle cavity. From here it moves anteriorly to pass over the anus and urinary pore before exiting through the exhalant siphon (formed of the right nuchal lobe).
The narrow, elongate hypobranchial gland is slender yellowish-white band on the left edge of the rectum (Fig 5, 11). Most of the mucus remaining in the mantle cavity of your specimen is probably still attached to this gland, from which it was secreted. Other mucus-secreting gland cells are scattered over the floor of the mantle cavity.
The rectum is a large tube lying immediately to the right of the hypobranchial gland (Fig 5, 11, 12-14B). It emerges from the left side of the visceral mass and runs obliquely across the right side of the roof of the mantle cavity. When it contains feces it is dark and easily seen and recognized. The empty rectum of a starved animal is less obvious. When feces are present, the rectum is a dark gray, brown, or black tube, the color ultimately depending on diet. The extreme anterior end of the rectum is not attached to the mantle and forms a short, free-hanging rectal papilla with the anus at its tip. This papilla can be seen on the right just inside the mantle cavity. The anus lies in the outgoing stream of water exiting the mantle cavity on the right through the exhalant siphon.
Find the thin-walled, transparent, large-diameter, tubular ureter (= urinary sinus) paralleling and adjacent to the rectum on the right side of the rectum. It is easier to see in males than in females, in which it is difficult to distinguish from the similarly thin-walled membranous uterus. The ureter runs from the nephridiopore of the kidney, in the far posterior end of the mantle cavity, to a large urinary pore near the anterior border of the right side of the mantle cavity. The urinary pore in males is to the left of the anal papilla and a little posterior to it. In females it is between the bases of the rectal papilla and the much larger, but similar, vagina (discussed below). Insert the tip of a standard, bent teasing needle into the pore and push it gently up the ureter for a short distance. This is the best way to find the ureter in females. Flow through the urinary pore is regulated by a sphincter muscle. Both anus and urinary pore release wastes into the exhalant current.
The ureter is not part of the ancestral metanephridium. Instead it is a device derived from the mantle epithelium to transport urine from the nephridiopore in the posterior mantle cavity to the exhalant siphon in the anterior cavity. It ends at the urinary pore which is not on a papilla. Being a freshwater animal inhabiting a hyposmotic environment, Bellamya produces copious dilute urine requiring a large diameter duct for its transport.
In females the uterus is a conspicuous feature of the right side of the mantle cavity (Fig 11). It is a thin-walled, large-diameter, transparent tube lying to the right of the ureter and rectum. It is typically filled with several large juvenile snails and many smaller embryos. It extends along the right side of the mantle cavity to a position near the mantle collar. Here it narrows to become the muscular tubular vagina which is hangs from the mantle roof, as does the rectal papilla. The vagina is the larger of the two. The female gonopore is at the tip of the vagina. The vagina regulates the exit of juveniles from the ureter and receives the penis during copulation.
Look now at the floor of the mantle cavity. The floor is the dorsal body wall covering the anterior visceral mass and cephalic hemocoel immediately posterior to the head.
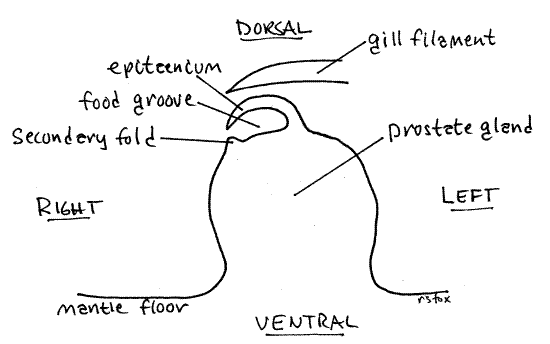
The topography of the floor is dominated by a feeding groove complex, which extends obliquely the length of the mantle cavity. It consists of two ridges with a groove between them. This complex begins in the posteriormost apex of the mantle cavity and extends anteriorly to the right side of the cavity to end between the right nuchal lobe and the right tentacle. Several functions have been attributed to this complex including channeling the flow of water through the mantle cavity, protecting the gill filaments from crowding by developing embryos, and filter feeding. It is similar, and presumably homologous to the epitaenium (epi = upon, taen = ribbon) of the amphibious ampullariid snails. In ampullariids the epitaenium channels water to the gill during aquatic respiration and to the lung during aerial respiration. Viviparids, however, are not amphibious and have no lung, so it seems unlikely that its chief function lies in channeling water to or away from the gill.
It is probably a feeding mechanism for collecting and transporting food particles to the mouth and it is described here in that context. It consists of a large sharp ridge of tissue on the left, which is the epitaenium (= branchial fold), and a much smaller, low, rounded secondary fold paralleling it on its right (Fig 6). Mucous gland cells are concentrated in a line along the base of the secondary fold. Between the two ridges is a shallow broad food groove lined with a ciliated epithelium. The thin crest of the epitaenium curves to the right and overhangs the food groove (Fig 6). It borders the food groove on the left. The groove is bordered on the right by the secondary fold.
Figure 6. Cross section of the epitaenium and food groove of a male. Gastrop72L.gif
In males this entire apparatus extends along the top of the large prostate gland for much of its length. The prostate extends about two thirds of the length of the mantle cavity but the epitaenium runs for its entire length. In females the epitaenium occupies the same position and is large and conspicuous, even though it is not on top of a prostate gland.
Anteriorly the epitaenium ends on the right side of the base of the right tentacle. The secondary fold and food groove, however, continue on as the cephalic food groove, exiting the mantle cavity to curve ventrally around the right tentacle to end ventral to the snout (Fig 1). The secondary fold is the right wall of the cephalic groove and the tentacle base is its left. The cephalic food channel is clearly visible as a curved ridge in ventral view of living snails crawling on the walls of aquaria.
The feeding apparatus for Viviparus viviparus has been described in the literature and is anatomically similar to that of Bellamya japonica. In V. viviparus the equipment is used for filter feeding and it seems cleat that is its purpose in Bellamya also. In Viviparus suspended food particles enter the mantle cavity with the respiratory current. The particles are caught on the gill filaments, mixed with mucus and moved by frontal cilia to the free tips of the filaments, where they drop off onto the ciliated floor of the mantle cavity. The filaments extend from the central axis to the right so their tips are located over the food groove on the floor of the mantle cavity (Fig 6). Particles that fall to the right of the secondary groove are caught in ciliary currents and moved to the left, over the secondary fold and into the food groove whereas particles that fall on or to the left of the epitaenium are moved to the right over the epitaenium and into the groove. Once in the groove, particles and mucus are moved anteriorly by its ciliated epithelium. A mucous string with entrained food particles moves anteriorly into the cephalic food groove curving around the right tentacle to the base of the snout where it accumulates. The snout periodically bends and the mouth ingests the mucous mass. The feeding apparatus has no provisions for sorting.
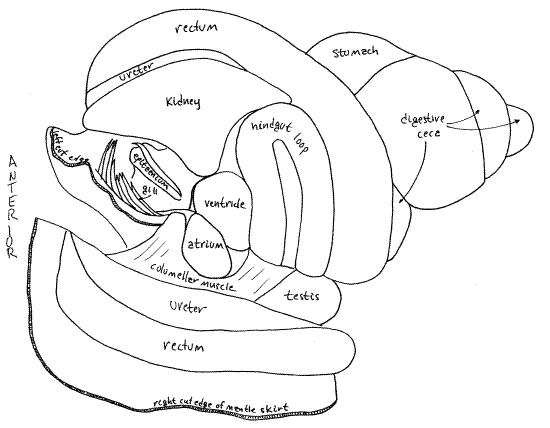
Figure 7. View of the area between the body and second whorls showing the apex of the opened mantle cavity and nearby organs. The animal has been rolled a little to the left, in comparison with Fig 5, to bring the right side into view. The pericardial cavity has been opened. Gastrop73L.gif
> e. To demonstrate particle transport and formation of the mucous string by the feeding apparatus you or your instructor may make a preparation of a freshly extracted living specimen that has not been anesthetized. Use method 2 in the Relaxation and Anesthetization section but immerse the snail in water, not alcohol. Pin the operculum to the wax bottom of the dissecting pan and open the mantle cavity with an oblique incision as described above, along the border between the rectum and gill. Mix carmine particles with condensed milk to make a thick pink suspension. You don’t need much. Place a drop of this suspension on the epitaenium and, at about 30X, watch ciliary currents move the milk and carmine particles transversely into the food groove where a mucous string of particles forms. The pink string with its entrained particles then moves anteriorly along the food groove into the cephalic food groove and then to the mouth. Let the preparation sit for 10-15 minutes then inspect the food groove where you will find a distinct cord of milky pink mucus extending into the cephalic food groove toward the mouth. Its movement is slow but discernable. <
In males the epitaenium sits atop a large rounded ridge, the prostate gland (= the seminal vesicle). The prostate extends obliquely from far posterior in the mantle cavity to a position at the base of the right tentacle (which is also the penis). In females there is no equivalent of the prostate gland and the epitaenium and food groove sits on the floor of the mantle cavity.
Excretory System
The excretory system of most prosobranchs consists of a single kidney (= nephridium) homologous to the left metanephridium of the pair present in the ancestral gastropods (Fig 12-14B). The right kidney is not present as such although vestiges remain.
Figure 8. The kidney, pericardial cavity, and heart. A portion of the hindgut loop is drawn as if transparent to reveal the ventricle. Gastrop90L.gif
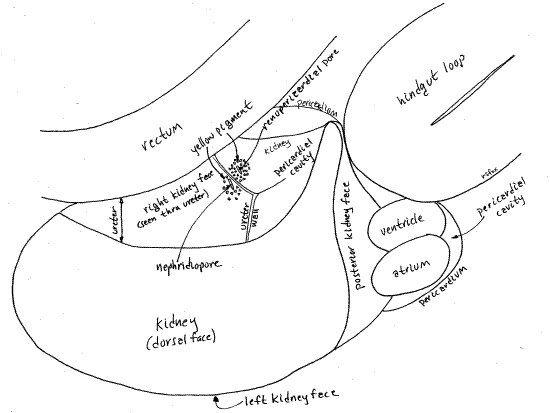
Without dissection, the kidney of Bellamya can be seen as a large, triangular, pale organ on the surface of the visceral mass immediately posterior to the posterior apex of the mantle cavity (Fig 5). It lies between the rectum and the conspicuous hindgut loop visible dorsally on the second whorl of the visceral mass. Its left surface forms the posterior wall of the mantle cavity so the kidney can also be seen from inside the mantle cavity by looking at the posterior end of the cavity.
The kidney is more or less pyramidal with four triangular sides. Its dorsal side is visible on the surface, facing you. The right side faces the ureter and the left side faces the mantle cavity. The posterior side faces the hindgut loop and the pericardium. The ureter and renopericardial aperture are on the right side. The latter will be discussed in connection with the hemal system.
" Extend the incision in the mantle roof posteriorly, following the right edge of the gill (Fig 13, 5). You will reach a place where the open area ends and your scissors encounter the kidney in their path. Let the incision veer to the right, following the left border of the rectum and the right border of the kidney. The incision will open the upper, blind end of the ureter.
The kidney protrudes into the posterior end of the mantle cavity and divides this part of the cavity into the right side (with nephridiopore and ureter) and the left side (with gill and epitaenium). The kidney lies on the left of the incision and the rectum on the right (Fig 5, 7, 13).
The nephridiopore opens from the right side of the kidney into the broad, blind end of the ureter which fits tightly against the right side of the kidney (Fig 9). The nephridiopore is on the right surface of the kidney facing the rectum but can be difficult to demonstrate. Examine the middle of the right side of the kidney with about 20X magnification and careful focusing. It is an oval slitlike aperture beside the posteriormost wall of the ureter. In fresh specimens it may have granules of yellow pigment scattered around it (Fig 9). Visualization of the nephridiopore is improved by pressing gently on the kidney with forceps while focused on where you think the pore should be. The pressure will force a cloud of particles from the pore.
The upper end of the long, wide, tubular, transparent ureter surrounds the anterior half of the right side of the kidney and nephridiopore, from which it receives urine. The ureter then extends along the roof of the mantle beside the rectum to empty through the small urinary pore just to the right of the anus. You have already seen the ureter and urinary pore. In females the urinary pore is between the rectal papilla and the vagina.
" Use your fine scissors to make an incision to the left of the kidney. This incision will begin at the anterior tip of the kidney and extend along the left side of the kidney, between the kidney and gill. It will open the left side of the posterior end of the mantle cavity. Use a pipet to wash away the accumulated mucus and debris. The left side of the mantle cavity is occupied by the gill and epitaenium. The ureter, you remember, occupies all the space in the right side of the posterior end of the mantle cavity.
The kidney is a large metanephridium and, as such, is a hollow sac with thick secretory and absorptive walls surrounded by hemocoel. One of the triangular sides of the kidney (the posterior side) faces the pericardial cavity. The oval renopericardial aperture connects the pericardial cavity with the lumen of the kidney. The aperture is on the posterior right wall of the kidney facing, and penetrating, the pericardium (Fig 8, 9, 10).
An ultrafiltrate of the blood is formed by the pericardial glands in the wall of the atrium and released into the pericardial cavity. It flows through the renopericardial aperture into the kidney lumen where it is modified by exchange with blood in the surrounding hemocoel (Fig 12-52). Here it becomes the final urine which then passes out the nephridiopore into the ureter. The urine is transported the length of the mantle cavity by the ureter which, as you know, opens under the mantle collar on the right side. Urine is carried out of the mantle cavity by the exhalant respiratory current.
Hemal System
" Return to the end of the incision on the left of the kidney. Extend this incision between the kidney and the hindgut loop (Fig 8, 7). Your scissors will cut through the thin, transparent, membranous pericardium and open the pericardial cavity. The cavity is nestled in the space between the posterior side of the kidney beside and underneath the hindgut loop. It also extends around the posterior right corner of the kidney to include the posterior end of the right side of the kidney (Fig 9).
The pericardial cavity is a coelomic space separated by the pericardium from the hemocoel. It is connected with the kidney by the renopericardial aperture (Fig 9). This aperture connects the anterior pericardium with the posterior end of the right wall of the kidney. In fresh specimens the aperture is surrounded by a ring of yellow pigment granules. It resembles the nephridiopore and, in fact, the two are very close together but on opposite sides of the wall of the upper end of the ureter.
Figure 9. Detail of the posterior right corner of the kidney and the associated structures. Gastrop184L.gif
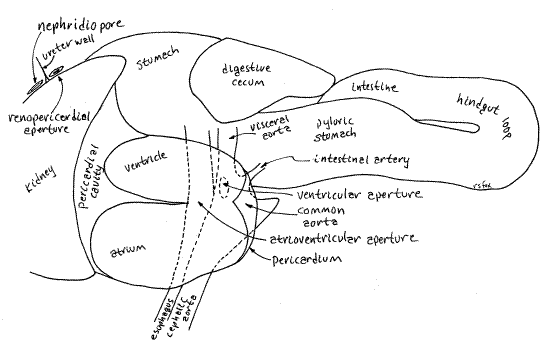
The pericardial cavity contains the heart consisting of two chambers, a glandularatrium (= auricle) and a muscular ventricle. With fine scissors open the pericardium to reveal the heart (Fig 7, 10). The walls of the atrium are thick and contain the podocyte-laden pericardial gland responsible for the formation of ultrafiltrate (Fig 12-118). The atrium is closest to the gill, from which it receives oxygenated blood via the large efferent branchial vessel. The ventricle is larger than and posterior to the atrium. It is adjacent to the anterior side of the hindgut loop whereas the atrium is adjacent to the posterior end of the mantle cavity and gill. The ventricle is hidden by the loop. The ventricle pumps oxygenated blood to the body.
The short common aorta exits the ventricle and immediately divides to form the cephalic aorta to the cephalic and pedal hemocoels of the head and foot respectively and the visceral aorta (= hepatic aorta) to the visceral hemocoel of the visceral mass. The venous return to the heart consists of several ill-defined blood sinuses channeling blood to the kidney, from which a well defined afferent branchial vessel takes it to the gill. The efferent branchial vessel drains the gill to the atrium (Fig 12-52).
" With your fine scissors open the atrium. Note the texture of the walls and look for the efferent branchial vessel entering the atrium from the gill. The atrium empties into the ventricle through the atrioventricular aperture (Fig 8, 10).
" Open the ventricle. The very short, but very wide, common aorta exits the posteroventral corner of the ventricle (Fig 10) through the oval ventricular aperture. The aperture is guarded by a thin membranous ventricular valve to prevent the return of blood to the ventricle during diastole. This aperture is the only exit from the heart. The common aorta divides immediately into the cephalic aorta and the visceral aorta. The cephalic aorta passes anteriorly just ventral to the floor of the mantle cavity and gives off several arteries to supply the head and foot. The visceral aorta supplies the visceral mass.
Figure 10. Detail of view in Fig. 8 showing heart and aortae. The hindgut loop and stomach have been deflected to the right. Gastrop91L.gif
Reproductive System
Most viviparids, including Bellamya, are gonochoric with copulation, internal fertilization, direct development, and viviparity. Large, yolky eggs are gestated lecithrophically (nourished by yolk and albumen) in the uterine region of the oviduct and become small snails before being released one by one through the vagina. Sexual dimorphism is slight but females are larger than males and the male right tentacle is thicker and distally hooked, unlike its unmodified counterpart in the female.
In general, prosobranch reproductive systems consist of a gonad whose products are led to the exterior by a gonoduct. The gonad, either ovary or testis, is located in the visceral mass beside the digestive cecum (Fig 12-14B). The upper, proximal region of the gonoduct leads from the gonad to the mantle cavity and is derived from the ancestral gonoduct and part of the right kidney duct. It is slender and unspecialized. The lower, distal portion of the gonoduct crosses the mantle cavity, is derived from mantle epithelium, and is known as the pallial gonoduct. It is typically composed of several regions with specialized functions.
Figure 11. Dorsal view of an undissected female. The shell has been removed. The head is retracted and the foot folded. Gastrop87L.gif
Female
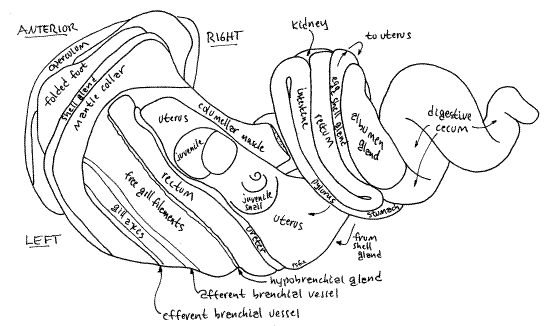
The female reproductive system consists of an ovary, oviduct, albumen gland, egg shell gland, uterus, vagina, and gonopore. The ovary is small, consisting of a few follicles embedded inconspicuously in the digestive ceca near the stomach. The upper oviduct exiting the ovary is also small and, like the ovary, probably will not be seen. It passes by the floor of the pericardial cavity where it is joined by the duct of the albumen gland. The lower gonoduct, known as the pallial oviduct, is much easier to see and includes the albumen gland, egg shell gland, uterus, and vagina.
The albumen gland is a large, beige, secretory gland adjacent to the upper end of the rectum (Fig 11). (The color of the albumen gland varies with species.) It is a side branch of the oviduct and connects with it by a short albumen gland duct. The oviduct exits the ovary, receives the duct from the albumen gland, and then widens to become the egg shell gland. The albumen gland secretes albumen, which sustains the developing embryos and juveniles. Albumen and yolk are the only known sources of food for developing embryos during gestation.
Soon after exiting the ovary and receiving the albumen gland duct the oviduct expands to become the narrow, elongate, pale yellowish egg shell gland (= seminal receptacle ( Fig 11). The egg shell gland is visible on the surface of the visceral mass in the groove between the albumen gland and the rectum. It secretes the thin transparent egg membrane (Fig 12) and probably also serves as a seminal receptacle for the storage of sperm. The membrane is secreted around an embryo and a supply of nutritive albumen, which supports the growth of the embryo in the uterus. Part of the membrane is twisted into a slender extension.
The egg shell gland increases gradually in width as it proceeds downstream and then abruptly expands to become the thin-walled, gray, transparent uterus that lies beside the rectum in the roof of the mantle cavity (Fig 11). It extends the length of the mantle cavity and, upon reaching the mantle collar, decreases in diameter and diverges from the mantle roof as the vagina. The tubular vagina hangs into the mantle cavity from the mantle roof. The vagina is muscular and regulates the release of juveniles from the uterus to the world outside.
" With your fine scissors open the uterus with a longitudinal incision extending its entire length. It will be filled with juvenile snails in various stages of development surrounded by a milky white fluid. With a pipet wash the fluid out of the uterine lumen so you can see the juveniles.
> f. Remove a few of the juveniles, selecting representatives of different sizes and examine them with the dissecting microscope (Fig 12). Each juvenile is enclosed in an egg membrane(secreted by the egg shell gland). If the adult is alive, the juveniles will be also and the larger ones will probably survive if transferred to an aquarium. <
Figure 12. Three developing juveniles from the uterus. Age increases left to right. Gastrop88L.gif
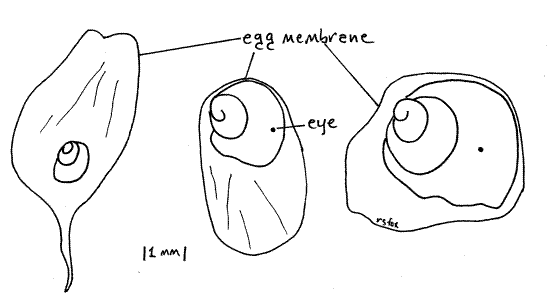
> g. Count the total number of juveniles present in the uterus of your specimen and record the data, along with the height of the mother’s shell (in millimeters), on the laboratory whiteboard. Compare your data with those recorded by other students. Is there a positive relationship between shell height of the mother and number of offspring in the uterus? The uterus of a 55-mm female dissected for this study contained 36 juveniles in various stages of development. All, even the largest (7 mm in diameter), were enclosed in an egg membrane. <
Remove all the remaining juveniles from the uterus. Clean the inside of the empty uterus thoroughly with squirts of water from a pipet and then examine its walls. A pair of longitudinal, side by side sperm ridges extends the length of the uterus from shell gland to vagina. One of the ridges is thin and convoluted with a sharp edge whereas the other is thick, only weakly convoluted, and has a blunt, rounded edge. Together these two ridges form a protected channel by separating the uterine lumen into a large gestation chamber, in which you found juvenile snails, and a smaller sperm channel. The sperm channel is the protected space between the two ridges and is an unobstructed path for sperm headed upstream from the vagina to the shell gland. During copulation the male inserts the right tentacle (penis) into the female's vagina and deposits sperm, which then move up the sperm channel to the shell gland (= seminal receptacle) where it is stored until used for fertilization of ova moving downstream from the ovary.
> h. Remove a small piece of shell gland and tear it to pieces in a drop of water on a slide. Affix a coverslip and examine the preparation with high power (400X) of the compound microscope. Look for active sperm. <
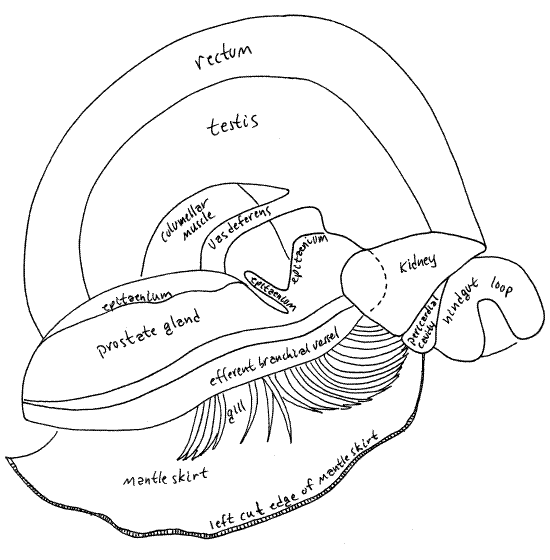
Male
The male reproductive system consists of a testis, vas deferens, prostate gland, sperm duct, and male gonopore. The sperm duct is in the penis and the gonopore is at the tip of the penis. The prostate gland and sperm duct constitute the pallial gonoduct, being derived from mantle epithelium.
The testis is a large, thick, pale cream-colored, crescent-shaped organ curving around the upper body whorl and lower second whorl of the visceral mass (Fig 5). The rectum lies on one side of it. (In some viviparids it is divided into two lobes and its color varies with species.) Unlike the ovary, it is large and conspicuous, easily visible on the surface. Its position corresponds to that of the female uterus. The testis makes almost a complete circle around the body and second whorls. Its anterior end lies to the right of the posterior mantle cavity and is clearly visible without dissection. The posterior end is adjacent to the right side of the kidney but is deeper in the visceral mass where it can be seen by pushing the second and third whorls apart.
The vas deferens arises through the confluence of numerous tiny vasa efferentia near the posterior (upper) end of the testis. The vas deferens is a short white duct extending across the surface of the columellar muscle (Fig 13). It is visible on the floor of the midregion of the mantle cavity before it enters the posterior (upper) end of the prostate gland, a little anterior to the kidney.
The prostate gland (= seminal vesicle) is a wide long hump on the floor of the mid and anterior mantle cavity. It is a thick cylinder extending obliquely across the floor of the mantle cavity. It is a pale, nearly white mound tapering to its anterior end at the base of the right tentacle. The epitaenium and food groove, run along its crest (Fig 6).
The short, tiny sperm duct extends from the tapered anterior end of the prostate through the penis (= right tentacle) to the male gonopore at the tip of the penis. The gonopore is difficult to see but can be demonstrated by squeezing the tentacle to extrude a thin white stream of semen. You can also hold the tentacle with your fine forceps so the tip is pointed up, toward you, and focus on the tip with about 30X. You probably can't see the pore like this but you can slip the point of a microneedle into it. Probe gently around the tip of the tentacle until the needle slips into the pore.
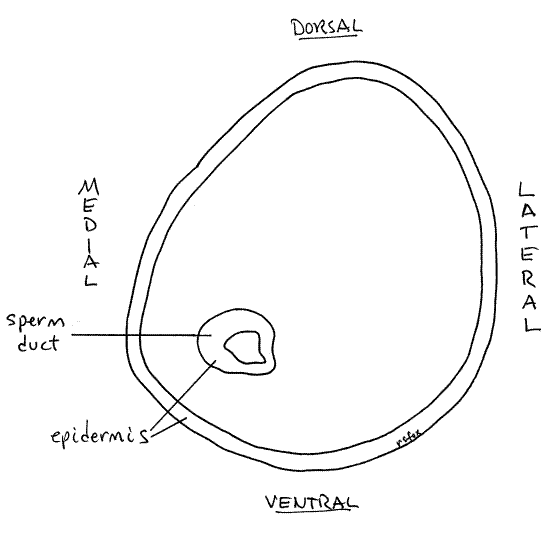
" After demonstrating the gonopore by one or both of the above techniques, use fine scissors to cut transversely across the right tentacle about midway along its length. Use 40X magnification to examine the stump of the tentacle. The sperm duct is easily seen in the cross section near the medial border of the tentacle (Fig 14). During development the pallial gonoduct begins as a ciliated groove in the mantle epithelium which then closes to become a tube.
Figure 13. Dorsal view of the midregion of the floor of the mantle cavity of a male Bellamya. The mantle cavity roof has been cut and reflected. Gastrop75L.gif
Digestive and Nervous Systems
Because most of the central nervous system is intimately associated with the anterior end of the gut, it is expedient to study the two systems simultaneously.
Anterior Gut
The gut comprises foregut, midgut, and hindgut. The foregut consists of the mouth, buccal cavity, and esophagus. As usual, initial food processing, including mechanical and some chemical digestion, occurs in the foregut. The midgut consists of the stomach and its diverticula, the digestive ceca. Most enzyme synthesis, hydrolysis, and absorption occurs in the midgut. The hindgut consists of the intestine, rectum, and anus. The hindgut is responsible for feces storage and formation of fecal pellets.
Figure 14. Cross section of the right tentacle (penis) of a male Bellamya. Gastrop89L.gif
" If you have not already done so, anchor the snail in place with its dorsal surface uppermost by forcing two # 7 stainless steel insect pins through the operculum to hold it (the operculum) flat against the wax of the dissecting pan. Insert one blade of a pair of fine forceps into the mouth and begin an incision along the mid-dorsal line of the snout. Extend the incision, which will open the anterior gut, to the base of the snout but no further at present.
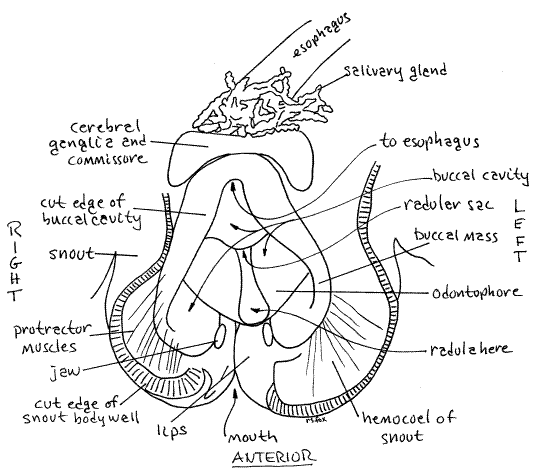
The anteriormost region of the gut, and the part opened by your incision, is the buccal cavity. It is enclosed in a complex of muscles known as the buccal mass (Fig 15). You incision cut through the thin upper wall of the buccal mass and opened the buccal cavity (= pharynx). The gut walls are thin and membranous. The muscles of the buccal mass operate the radula and its supporting odontophore, which are used in a type of feeding know as microphagous browsing. Some of the muscles extend from the mass to the nearby body wall (extrinsic muscles) but some are confined to the mass (intrinsic muscles). Two jaws are visible as a pair of small, yellow, chitinous plates, one on each side of the anterior buccal cavity (Fig 15).
The conspicuous radula lies on the floor of the buccal cavity, posterior to the jaws. It consists of parallel rows of teeth extending posteriorly out of sight. You will study the radula in more detail later but at present increase the magnification to about 40X and take a closer look at it and its abundant teeth (Fig 16). The radula emerges from a pocket in the floor of the buccal cavity known as the radular sac.
" From the base of the snout continue the mid-dorsal incision posteriorly but now it should be no deeper than the thickness of the gray integument. This cut must be shallow to avoid destruction of the nerve ring, which lies immediately under the skin and encircles the anterior gut. It should not open the gut.
The body cavity opened by this incision is the cephalic hemocoel. The buccal mass is surrounded by this hemocoel which receives blood from the cephalic aorta, one of the two branches of the common aorta exiting the ventricle of the heart.
The two cerebral ganglia of the nerve ring lie dorsal to the gut and a little lateral to the midline. In fresh specimens the ganglia are bright red or reddish brown, making them easy to recognize. The color is due to the oxygen-storing molecule, neuroglobin. The ganglia are connected with each other by the cerebral commissure (Fig 15, 17, 12-17, 12-53). Together the two ganglia are the brain. The commissure is a transverse band of neurons that crosses the midline to connect the two ganglia. It may have been cut if the incision was a little too deep. Several large yellow (fresh) or white (older) nerves can be seen entering (sensory) or leaving (motor) the cerebral ganglia. Among them are sensory nerves from the tentacles and eyes and motor nerves to the buccal ganglia and snout which control the myriad muscles that make up the buccal mass and operate the radula. During your dissection of the digestive system try to avoid damaging nerves or blood vessels if you plan to study these systems later.
Figure 15. Dorsal view of the buccal mass and anterior gut. Most of the nervous system has been omitted for clarity. The buccal cavity has been opened by a median dorsal incision and the radula has been removed. Gastrop76L.gif
A small red buccal ganglion is present on each side of the buccal mass, anterior to (and much smaller than) the cerebral ganglia. It will probably be hidden under the curled edge of the gut wall if the gut has been opened. The buccal ganglia are connected to the cerebral ganglia by very long recurved cerebrobuccal connectives and to each other by the buccal commissure (Fig 17, 12-53). The cerebrobuccal connectives are free in the hemocoel, lateral to the buccal mass, for most of their length. The connective passes anteriorly lateral to the buccal mass then reverses direction and enters the buccal mass, penetrating its muscles to reach the buccal ganglion. The buccal commissure emerges from the muscles of the buccal mass and extends transversely across the midline ventral to the buccal cavity but dorsal to the radular sac.
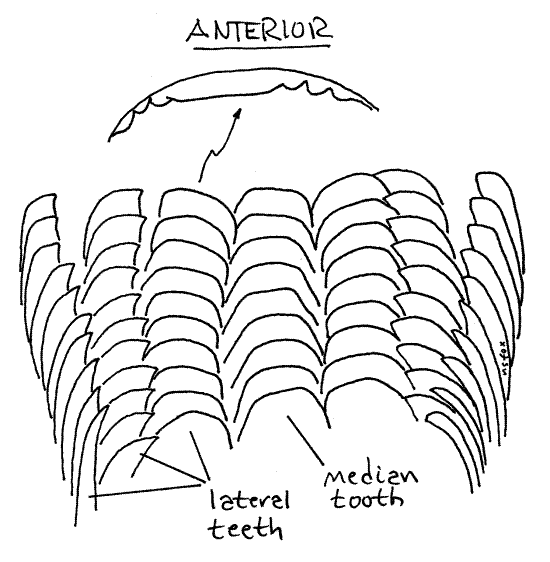
Relocate the radula. Gently grasp its tip and pull it a few millimeters anteriorly. Do not pull it all the way out of the buccal mass yet. Look at the radula with higher magnification, about 30-40X. The longitudinal ridges evident on its surface are actually rows of chitinous teeth. Count the rows. There are seven of them. Focus carefully on the teeth at 40X and note that their cutting edges bear fine tooth-like denticles.
The radula (Fig 12-2A) is a renewable, constantly growing, constantly eroded, filelike ribbon of chitinous teeth emerging from a pouch, the radular sac, opening on the floor of the buccal cavity (Fig 12-53). Its position resembles that of a tongue. The sac in Bellamya is a short, longitudinally oriented, blind pouch that extends posteriorly ventral to the buccal cavity and esophagus. Over most of its length its lumen is separated from that of the buccal cavity by a transverse horizontal septum. In Bellamya the radula is short but well developed. It is only about one fifth of the shell height whereas that of many prosobranchs exceeds shell height.
Figure 16. Part of the anterior end of the radula of Bellamya at 125X magnification. The denticles of the cutting edge of one left lateral tooth are shown at higher magnification (500X). gastrop93L.gif
The radula sits on an oval pad of dense connective tissue known as the odontophore (odont = tooth, phore = to bear). The radula lies in a median longitudinal trough in the odontophore, which can be seen extending laterally on both sides of the radula.
Mesogastropods, such as Viviparus, have taenioglossate radulae (Fig 12-40C) characterized by having seven longitudinal rows of teeth. In other words, each transverse row consists of seven teeth. A median tooth in the center is flanked by three teeth on each side. Count the tooth rows to verify this.
The radula and the odontophore are operated by the numerous muscles of the buccal mass (Fig 12-14B). Most muscles extend from the buccal mass to the nearby body wall (extrinsic muscles) but some are confined to the mass (intrinsic). Some of the extrinsic muscles are radula or odontophore protractors and others are retractors. Protractors extend anteriorly from the buccal mass whereas retractors extend posteriorly. Look for examples of these.
> i. With fine forceps carefully pull the radula out of the radular sac and make a wetmount for examination with the compound microscope. Count the number of teeth in a transverse row. Confirm the presence of seven teeth in each row. There is a median tooth (= rachidian) in the center with three lateral teeth on each side, for a total of seven. The outer most lateral teeth are sometimes known as marginal teeth. Notice that the cutting edges of the teeth bear tiny denticles along their cutting edges. <
> j. With a centimeter rule measure the length of the radula and compare it with the height of the shell. The Bellamya radula is proportionately much shorter that that of most prosobranchs, including other viviparids, presumably because it relies, at least partly, on filter feeding instead of microphagous browsing for nutrition. The radula of Viviparus viviparus, for example, is reported to be 2.5X the shell height and has about 70 transverse tooth rows. <
" Extend the middorsal incision from the anterior end of the head posteriorly, through the floor of the anterior mantle cavity through the epitaenium. This incision expands your access to the cephalic hemocoel, one of the major sinuses of the hemal system and part of the functional body cavity of the snail. A branch, the pedal hemocoel, extends into the foot. Stop the incision here for now. In males this incision necessitates cutting through the thick cylindrical prostate gland on the floor of the mantle cavity. Notice that the prostate has thick glandular walls with a relatively small lumen.
> k. If your specimen is a male, use a plastic Pasteur pipet to remove some of the sperm from the interior of the prostate gland and make a wetmount with it. Examine the sperm with high power (400X) of the compound microscope and describe their shape. Two types of sperm are present in viviparids. Functional sperm are typical and have a spiral head and a single long flagellum. Atypical sperm are vermiform or spiral with numerous short flagella. <
Return to the anterior gut, continuing to be protective of the nervous system. The buccal cavity extends a short distance posteriorly from the opening of the radular sac. In the vicinity of the cerebral ganglia, while still in the buccal mass, it expands to become the esophagus which then exits the posterior end of the buccal mass.
The salivary glands are attached to the anterior end of the esophagus and form a tangled mass of slender lobulated tubules on the dorsal surface of the buccal mass and cerebral ganglia (Fig 15). Two salivary glands are present but their tubules form such a tangle that they cannot be separated.
Visualization of the thin-walled anterior gut is difficult. The opening to the radular sac, however, is easily seen and can be used as a landmark. The buccal cavity extends a short distance posteriorly from this opening. Carefully slip one point of your finest forceps into the space dorsal to the radular sac and ventral to the cerebral ganglia. The point will be in the buccal cavity and will be clearly visible through the thin, transparent gut wall where it is not overlain by opaque tissues such as the brain or salivary glands. Of course, you must be careful using this technique that you follow the gut lumen and do not force the point through the wall of the gut. Be sure you do not accidentally slip the point into the radular sac instead of the buccal cavity. The point will pass under the cerebral ganglia and commissure, into the esophagus, and under the salivary glands to appear posterior to the brain.
Nervous System
Before tracing the esophagus and posterior regions of the gut, return your attention to the central nervous system. Bellamya is a good species for the study of the gastropod nervous system. It exhibits most of the features of a typical molluscan nervous system but shows the effects of torsion characteristic of gastropods. The system is exposed in the cephalic hemocoel and most of it can be seen with little further dissection. The ganglia of the CNS are usually red in fresh specimens.
" Use fine forceps and scissors to remove as much as possible of the salivary glands to reveal structures ventral to them. Be careful to avoid damaging nerves near the glands.
The mollusc central nervous system consists of several pairs of ganglia joined by nerve cords, either commissures or connectives (Fig 17, 12-53). The major ganglia are the paired cerebral, buccal, pedal, pleural, esophageal (= intestinal or parietal), and the unpaired visceral ganglia. Commissures are transverse connections between paired ganglia. Connectives, on the other hand, are cords between ganglia of different pairs and they are usually longitudinal. With the exception of the visceral, the ganglia are concentrated in the vicinity of the anterior esophagus where some of them form a nerve ring around the gut.
The nerve ring encircles the anterior esophagus and consists of the paired cerebral and pedal ganglia joined to each other by their commissures and connectives (Fig 17). Specifically these are: right cerebral ganglion, cerebral commissure, left cerebral ganglion, left cerebropedal connective, left pedal ganglion, pedal commissure, right cerebropedal connective, and then back to the right cerebral ganglion. Use a pencil to lightly follow the nerve ring on Figure 17.
The two large red cerebral ganglia lie dorsally on either side of the buccal mass between the bases of the cephalic tentacles. They are connected by the conspicuous cerebral commissure which passes transversely over the anterior esophagus in the buccal mass. The cerebral ganglion innervates the head, snout, tentacles, eyes, and statocyst. A large tentacular nerve exits laterally from the ganglion and extends to the cephalic tentacle (Fig 17). Slightly posterior to it is a much smaller optic nerve to the eye. Two nerves exit posteriolaterally and extend to the snout. A single long cerebrobuccal connective exits beside the two snout nerves but goes to the buccal ganglion.
The two buccal ganglia are on the dorsal surface of the buccal mass anterior to the cerebral ganglia. They are joined to the cerebral ganglia by the long, recurved cerebrobuccal connectives partially hidden in the muscles of the buccal mass. The wide, but relatively short, buccal commissure extends transversely to connect the two buccal ganglia. It crosses the midline ventral to the buccal cavity but dorsal to the radular sac. Each buccal ganglion sends two large motor nerves to the muscles of the buccal mass.
Figure 17. Dorsal view of the central nervous system of a male Bellamya. The buccal mass, esophagus, and odontophore have been removed for clarity. In life the esophagus would pass ventral to the cerebral commissure and dorsal to the pedal commissure. Note the effects of torsion on the visceral loop (supra- and sub-esophageal nerves). comm. = commissure, con. = connective, g = ganglion, n = nerve. Gastrop84L.gif
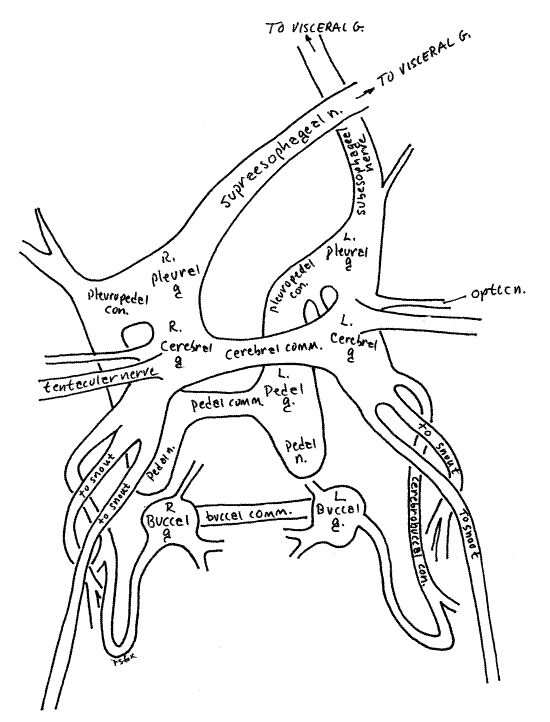
The spatial relationships between the cerebral, pleural, and pedal ganglia are not bilaterally symmetrical in Bellamya. The pleural ganglia are posterior and ventral to the cerebral ganglia and connected to them by a thick connective. The two pleural ganglia are not connected by a commissure. They are, however, closely associated with the pedal ganglia and the cerebropedal connectives. The pleural ganglia innervate the nuchal lobes, cephalic food groove, tentacle bases,
A long connective exits the each pleural ganglion and extends posteriorly through the hemocoel. Ultimately the two join the visceral ganglion (which consists of two fused visceral ganglia) in the visceral mass near the pericardial cavity. These connectives cannot be traced for their entire length without damaging the digestive system and tracing them, if it is done at all, should be postponed until you have finished the gut.
The connective from the right pleural ganglion is the supraesophageal connective that extends diagonally to the left by passing dorsal to the esophagus. Shortly after crossing the esophagus, it branches to send a nerve (the anterior branchial nerve) to the anterior gill and osphradium. The supraesophageal ganglion is a small swelling at the point of exit of this branch. The supraesophageal connective continues posteriorly along the left side of, and close to, the esophagus until it exits the cephalic hemocoel under the posterior end of the mantle cavity. The supraesophageal connective and ganglion innervate the mantle muscles, exhalant siphon, vas deferens and testis of the male, uterus and ovary of the female.
The left pleural ganglion gives rise to the subesophageal connective, which extends obliquely to the right by passing ventral to the esophagus. It then runs posteriorly along to the right of, and parallel to, the esophagus but not nearly as close to the esophagus as is the subesophageal connective. It also bears a ganglion, the subesophageal ganglion, and has branches to the mantle and epitaenium. It also ends up at the visceral ganglion. The subesophageal connective and ganglion send nerves to the mantle muscles, gill, and, osphradium.
These two connectives form the visceral loop between the pleural ganglia and the visceral ganglion (Fig 12-17). In most molluscs the connectives extend more or less straight posteriorly to arrive at the visceral ganglion under the kidney. In gastropods, however, torsion has twisted the visceral loop into a figure eight and the connectives cross each other, as described above, before reaching the visceral ganglion.
The visceral ganglion is composed of the two fused visceral ganglia. It lies near the kidney in the connective tissue at the posterior end of the cephalic hemocoel. It is formed by the junction of the two connectives of the visceral loop. The visceral ganglion innervates the heart, kidney, stomach, and hindgut. The visceral ganglion probably will not be seen.
The two elongate pedal ganglia are hidden deep below the esophagus and can only be glimpsed by pushing the buccal mass and gut a little to one side. The pedal ganglia send motor nerves to the muscles of the foot.
" A more extensive dissection, requiring removal of the buccal mass and anterior gut, will reveal the pedal ganglia fully but this should not be undertaken until after you have completed your study of the digestive system.
Each ribbon-like pedal ganglion is connected with a cerebral ganglion by a thick cerebropedal connective which is shared with a pleural ganglion. Ventrally the transverse pedal commissure connects the right and left pedal ganglia to complete the nerve ring. The pedal ganglia and cerebral ganglia are the ganglia of the nerve ring.
Peripheral nerves to and from the body extend from the ganglia and the larger ones are easily seen. The many nerves of the cerebral ganglia serve the sensory structures of the head including the skin, cephalic tentacles, statocysts, eyes, anterior buccal mass, and snout.
Sense Organs
Most of the sensory structures have already been seen. The cephalic tentacles are mechanoreceptors, sensitive to tactile stimuli, and are probably also chemoreceptive. The exposed body surface is also receptive to chemical and mechanical stimuli.
The paired eyes are photoreceptors, of course, but are not capable of forming an image. They are located on short ocular peduncles fused to the lateral margin of the cephalic tentacles. Early in the evolutionary history of prosobranchs the eyes occupied stalks independent of the tentacles as they do still in some archaeogastropods such as abalones. In higher gastropods the eyestalk is fused with the base of the tentacle and often leaves no trace of its former presence. In viviparids the eyestalk (= ocular peduncle) is still present. Each eye has a cornea, retina, and pigment cup and is connected with the cerebral ganglion by an optic nerve.
The osphradium is located in the inhalant respiratory water current where it can monitor water on its way to the gill. It is chemosensory but probably is also a mechanoreceptor capable of detecting silt. In Bellamya it is small and inconspicuous. It is innervated by the osphradial nerve from the supraesophageal ganglion.
The two statocysts are organs for the detection of gravity. Each is an oval vesicle situated lateral to a pedal ganglion. Each is in a deep pocket in the floor of the hemocoel but may be visible without further dissection after the esophagus has been removed. The vesicle contains a statolith composed of calcium carbonate granules which respond to a gravitational field. A sensory nerve extends to a cerebral ganglion from each statocyst.
Posterior Digestive System
" Trace the esophagus through the cephalic hemocoel under the floor of the mantle cavity. This will necessitate extending your incision through the floor of the mantle cavity. If the animal has fed recently the esophagus will be distinctively pigmented, usually green, depending on the food. The esophagus of starved specimens will be an empty, transparent tube.
After exiting the buccal mass the esophagus turns to the left and heads posteriorly, still in the cephalic hemocoel, under the floor of the mantle cavity and on top of the broad, white columellar muscle. It curves posteriorly to the right more or less under the path of the epitaenium. In males it will be under the seminal vesicle as well. Its appearance is usually that of a collapsed tube and it is enclosed in a tubular hemocoelic space (the venous return from the cephalic hemocoel). This space should be opened with fine scissors.
Unpin the snail from the wax and extend your incision through the floor of the mantle cavity all the way to the end of the mantle cavity. This incision will follow the esophagus to the end of the cephalic hemocoel and will require that you roll the snail to the right to follow the mantle cavity. Eventually the esophagus will empty into the stomach but that occurs high in the third whorl. After exiting the cephalic hemocoel the esophagus leaves the surface and takes a short cut, so to speak, passing up the core of the visceral mass along the columellar axis into the third whorl. It may be difficult to trace the esophagus along this part of its path. Upon reaching the third whorl the esophagus enters the left corner of the stomach (Fig 5).
The stomach, unlike the esophagus but like most of the remainder of the gut, is on the periphery of the visceral mass, rather than deep in its interior. Consequently much of it is clearly visible on the surface of the third whorl where you saw it earlier in the exercise (Fig 5). Postpone study of the stomach interior until you have traced the hindgut all the way to the anus.
Most of the length of the hindgut (intestine and rectum) is exposed on the surface of the lower whorls of the visceral mass. The stomach curves around to the right of the third whorl. The intestine exits the stomach after it (the stomach) has made almost a complete revolution around the axis. Roll the snail to the left and find the exit of the intestine from the stomach. The hindgut makes two tight loops, the hindgut loop, after exiting the stomach (Fig 11). The hindgut is entirely visible on the surface and can be traced easily by carefully snipping the connective tissue holding it to the surrounding tissues. The intestine exits the pyloric end of the stomach, makes a hairpin loop on the left side of the dorsal surface of the second whorl (Fig 11, 5, 18). Upon exiting this loop the midgut again passes across the ventral side of the second whorl to enter a second hairpin loop which it exits as the rectum (Fig 11, 18).
Figure 18. Another view of an intact, extracted animal for comparison with Fig 5. In this drawing the right side of the foot and anterior body whorl are shown. The coil of the second and third whorls are stretched and the upper whorls have been twisted to the right to reveal structures of the left side not visible in Fig 5. The foot is folded and the head is retracted. Arrows indicate the flow of food through the gut. Gastrop85L.gif
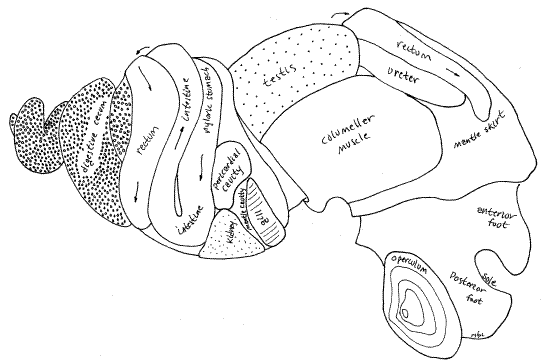
The rectum has a larger diameter and is much longer and more conspicuous than the intestine. The rectum, which has already been seen, exits the second loop (Fig 18) and extends left to right across the "front " or "dorsal" side of the second whorl (Fig 5), then extends right to left over the surface of the back of the second and body whorls to emerge on the roof of the mantle cavity on the body whorl (Fig 5). In males it follows the outside curve of the testis. Similarly in females it lies beside the shell gland and uterus (Fig 11). You have already traced the path of the rectum along the length of the mantle cavity roof and have found the terminal anus at the end of the rectal papilla on the right side of the mantle cavity.
" Go back now to the stomach in the third whorl. Use fine scissors to open the stomach with an incision from the esophagus through the length of the long tapering stomach to the upper end of the intestine. This incision will cut through the thin, membranous body wall and the stomach wall. Use a pipet to flush the contents of the stomach away so you can study the stomach walls. The stomach is divided into an upstream cardiac region (adjacent to the esophagus) and a downstream pyloric region (preceding the intestine (Fig 19). Cardiac and pyloric regions are separated by a short, thick, transverse ridge in the left stomach wall. The cardiac region itself is divided into smaller chambers and the opening of the duct from one of the digestive ceca (apical cecum) opens into its left wall under an overhang of tissue. The ducts of two other digestive ceca (right and left ceca) open in the pyloric region. A narrow longitudinal ridge of tissue, the typhlosole, arises in the downstream end of the pyloric stomach and extends into the intestine. The pyloric stomach ends in a small pouch under the overhang of a loop in the typhlosole of the pyloric stomach.
Figure 19. The stomach opened to reveal its internal structure. Arrows indicate the flow of food. Gastrop86L.gif
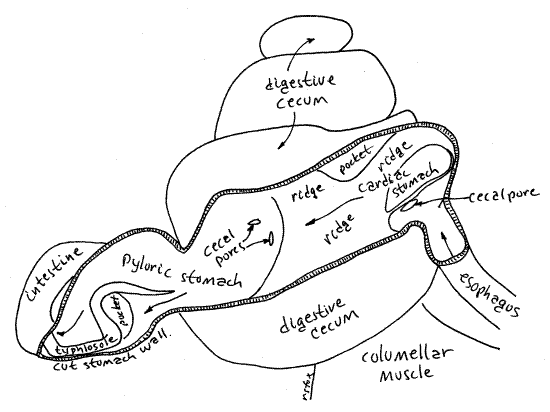
Three digestive ceca (= liver, digestive gland) are present and are the apical cecum, right cecum, and left cecum. Each is a hollow, highly branched diverticulum of the stomach and is connected with the stomach by a duct. The openings of these three ducts, the cecal pores, are visible on the stomach walls as described above. Their identity can be confirmed by gently pressing on the nearest cecum. Cecal contents will ooze from the pore.
The thick walls of the ceca consist of thousands of elongate pouches, or acini, of secretory and absorptive epithelia surrounding a lumen (Fig 12-103B). The ends of the acini are easily seen through the transparent body wall covering the digestive ceca. The acini are connected by a series of ducts ultimately leading to the cecal pores opening into the stomach.
" Extend the stomach incision into the upper end of the intestine. The intestine exits the downstream end of the pyloric stomach. The end of the pyloric stomach and the upper end of the intestine form the first half of the hindgut loop visible on the surface of the second whorl. Open the intestine and note that the strong typhlosole of the pyloric stomach becomes smaller and disappears in the intestine. It does not reach the second half of the hindgut loop. Immediately upon leaving the intestinal loop the intestine enters the second half of the loop in which the intestine is one arm and the upper rectum is the other.
" Extend the incision into the upper rectum and open it all the way to the anus. Use your pipet to clear the stored feces from its lumen. Note that the rectum has no typhlosole. The characteristic small oval fecal pellets of this species are formed as the feces are extruded from the anus.
References
Alante FH. 1930. Biology of Vivipara angularis Müller, a common fresh-water snail in Laguna de Bay. Philippine Agriculturist 19:307-325 .
Annandale N, Seymour Sewell RB. 1921. The banded pond-snail of India (Vivipara bengalensis). Rec. Indian Mus. 22:215-292, pls. 1-3.
Baker FC. 1928. The freshwater Mollusca of Wisconsin, Part I. Gastropoda. 507 p. Wisconsin. Aca. Sci. Arts Lett., Madison.
Brown KM. Mollusca: Gastropoda, pp 297-329 in Thorp JH, Covich AP (Eds.). Ecology and classification of North American freshwater invertebrates, 2 nd ed. Academic Press, San Diego. 1056pp.
Call RE . 1888. Gross anatomy of Campeloma. American Natur. 22:491-497, pl. 7.
Chang M. 1928. The anatomy of fresh-water viviparous snails. Peking Soc. Nat. Hist. Bull. 3:45-57, pls. 1-2.
Cook PM. 1949. A ciliary feeding mechanism in Viviparus viviparus (L.). Proc. Malacological Soc. London , 27:265-271.
Dillon RT. 2003. The freshwater gastropods of South Carolina. www.cofc.edu/~dillonr/FWGSC/b_japonica.html
Eckel PM. 2004. The oriental mystery mollusc (Cipangopaludina chinensis) at Buckhorn Island State park, Erie County, New York. www.mobot.org/plantscience/ResBot/niag.Misc/Mollusc/Mollusc.htm
Fretter V, Graham A. 1994. British prosobranch molluscs, their functional anatomy and ecology, 2 nd ed. Ray Society, London, 161:1-820.
Kohl M . undated. Freshwater molluscan shells: http://members.aol.com/Mkohl1/Viviparidae.html
Li F-C. 1935. Anatomie von Paludina. Chinese J. Zool. 1:1-18.
Mattox N. 1938. Morphology of Campeloma rufum. J. Morph. 62:243-258, pls. 1-2.
Pennak RW . 1989. Fresh-water Invertebrates of the United States, 3 rd ed. Wiley, New York. 628p.
Ruppert EE, Fox RS, Barnes RB. 2004. Invertebrate Zoology, A functional evolutionary approach, 7 th ed. Brooks Cole Thomson, Belmont CA. 963 pp.
Supplies
Dissecting microscope
Compound microscope
Dissecting set including fine forceps, fine scissors, blunt probe, scalpel, microneedles, and heavy forceps
Large viviparid snail
Small deep dissecting pan (e.g. tuna can with wax bottom)
2-inch C-clamp (or vise)
2 # 7 stainless steel insect pins
Compound microscope
Slides and coverslips
Carmine and condensed milk suspension made up in the fluid that surrounds
the dissected animal
Centimeter rule
Applicator sticks
Small rubber bands
Freezer
7% ethanol (non-denatured)