Invertebrate Anatomy OnLine
Bdelloidea ©
Moss rotifers
5jul2006
Copyright 2001 by
Richard Fox
Lander University
Preface
This is one of many exercises available from Invertebrate Anatomy OnLine , an Internet laboratory manual for courses in Invertebrate Zoology. Additional exercises, a glossary, and chapters on supplies and laboratory techniques are also available at this site. Terminology and phylogeny used in these exercises correspond to usage in the Invertebrate Zoology textbook by Ruppert, Fox, and Barnes (2004). Hyphenated figure callouts refer to figures in the textbook. Callouts that are not hyphenated refer to figures embedded in the exercise. The glossary includes terms from this textbook as well as the laboratory exercises.
Systematics
Gnathifera SP, Syndermata, Rotifera P, Bdelloidea C, (23-26, 9-26)
Gnathifera SP
Gnathiferans have a complex pharynx with a cuticular jaw apparatus operated by an elaborate pharyngeal musculature. Most are small, less than 1 mm. The taxon includes Rotifera, Gnathostomulida, Micrognathozoa, Acanthocephala, and Seisonida.
Syndermata
Rotifers, seisonidans, and acanthocephalans share a syncytial epidermis with an cytoplasmic skeleton known as the intrasyncytial lamina (Fig 23-12).
Rotifera P
Rotifera includes some of the smallest metazoans and most of the 2000 described species are less than 0.5 mm in length. Most are microscopic, freshwater, and free swimming. These tiny animals superficially resemble ciliate protozoans in size and behavior but are multicellular, with about 1000 cells each.
Rotifers are common animals that can be found in almost every conceivable freshwater habitat including such obvious locations as lakes and rivers but also in places that are moist only periodically, such as moss, soil, and leaf litter. They are the most abundant metazoans in freshwater benthic habitats and are one of the three dominant metazoan taxa in the freshwater zooplankton. Rotifers occur at all except polar latitudes. Most inhabit freshwater habitats although about 50 species are marine. Two genera are exclusively marine.
Rotifers have a fluid filled body cavity containing a loose syncytium of amoeboid cells. The body cavity is a hemocoel, or pseudocoel, derived from the blastocoel of the embryo. There is no mesothelium. These animals are eutelic and individuals of each species have a characteristic and constant number of cells. Tissues are often syncytial. The characteristic number of cells originates in the embryo and remains for life with growth occurring through increase in cell size, rather than through addition of new cells.
Rotifers have characteristic ciliary ring, or corona, near the anterior end to generate the feeding and locomotory current. The body wall consists of a syncytial epidermis, containing an intrasyncytial lamina, known as the lorica, and underlain by individual circular and longitudinal muscles. The lorica may be thin and flexible or thick and rigid. The gut is usually, but not always, complete and the muscular pharynx includes an elaborate, cuticular mastax with up to seven hard jaws, or trophi. Osmoregulation is via protonephridia. No special respiratory or hemal systems are present. Gas exchange is accomplished over the unelaborated body surface and fluid transport by diffusion and movement of the blood in the hemocoel.
The nervous system includes a dorsal cerebral ganglion, two pairs of longitudinal nerve cords, a mastax ganglion, and a caudal ganglion. The sense organs include eyes, bristles, pits, and antennae. Rotifers are gonochoric but, depending on taxon, males are rare, unknown, or produced only occasionally.
Reproduction is usually parthenogenetic but is sometimes sexual in those monogonont species with both sexes. Males, when present, are smaller than females, short-lived, aberrant or degenerate, and usually have no functional gut and do not feed (Fig 23-20A). Females have a gonad consisting of a combined germarium (ovary) and vitellarium (yolk gland). Descriptions of rotifer anatomy and biology, including this one, apply to females unless stated otherwise. Most rotifers are oviparous but a few planktonic species are viviparous.
Bdelloidea
Bdelloids inhabit freshwater and most are found in intermittently moist terrestrial mosses and soil but some are benthic in aquatic habitats. Those that inhabit terrestrial habitats are tolerant of desiccation. Male bdelloids have never been observed and are presumed to be nonexistant. Reproduction is always parthenogenetic. Females have two germovitellaria as opposed to the solitary one of Monogononta. The contractile, wormlike body is elongate and composed of 16 telescoping rings. The head is retractable and has a well developed corona with two trochal discs. The mastax is adapted for grinding.
Introduction
Bdelloid rotifers have the ability to undergo cryptobiosis and withstand extended periods of desiccation and return to metabolic activity when wetted. This ability makes it possible for them to inhabit bryophytes (mosses) in terrestrial habitats that are only periodically wet. During wet conditions the rotifers inhabit the thin film of water on the surface of the moss. Here they feed and complete the parthenogenetic life cycle. The well developed foot and toes maintains contact with the moss substratum. They are elongate and vermiform to facilitate maneuvering in the tiny spaces among the moss "leaves".
When the weather is dry, the film of water begins to dry and the rotifers start the process of forming a desiccation resistant "tun". This is not a cyst for there is no secreted covering. The tun is simply the dried rotifer enclosed in its wrinkled and withered integument (Fig 23-17B). The formation of the tun is not rapid and rotifers require the presence of interstices and crevices in which trapped water evaporates more slowly than from the surface of the leaves. Rotifers artificially deprived of these interstices will not survive the drying process.
With the return of wet conditions the rotifer absorbs water and resumes its normal morphology, metabolism, and activity. Rehydration can occur in as little as 10 minutes or may take up to a day.
Mosses are a convenient source of living rotifers for laboratory exercises. Mosses can be stored dry in a jar in the laboratory and wetted whenever rotifers are needed. Rotifers have been revived after 27 years sitting dry on a shelf. Alternately, the class can take a short walk on campus, collect fresh moss from suitable trees, and rehydrate the rotifers upon return to the laboratory.
Laboratory Specimens
This exercise provides a superficial study of rotifer anatomy utilizing locally collected specimens. For a more detailed examination of rotifer morphology see the Philodina exercise atInvertebrate Anatomy OnLine.
Bdelloid rotifers can usually be extracted from moss collected on campus. In the laboratory, place a small sprig of moss in a 9-cm culture dish and completely cover it with water. Spring water or lake water should be used. Deionized water is acceptable but do not use tap water. With a dissecting microscope examine the submerged moss for signs of activity. You probably will not see anything. Set the dish aside for 15-30 minutes have passed and look once more at the moss with the dissecting microscope. This time you should see active rotifers.
Using 40X find and watch a rotifer for a few minutes. Observe the way it uses the adhesive toes at the posterior end of the foot to attach to and move over the substratum. The rotifer may move in a leech-like looping manner in which it alternately attaches and releases the posterior and anterior ends.
Dishes that have been standing for several days may have numerous rotifers using the surface tension as a substratum. These rotifers are especially easy to observe as they move leech-like over the under side of the surface tension of the water in their dish.
Using 20X find a moss leaflet that has several rotifers on it. Use your microdissecting forceps to twist this leaf off the plant and transfer it to the center of a clean slide. Find a leaf with several rotifers on it and make a wetmount with it. You need more than one rotifer on your slide to compensate for the inevitable individuals that are on the wrong side of the leaf or otherwise unobservable.
Anatomy
Examine the slide with the scanning lens of the compound microscope to find a rotifer in an observable position. The larger the specimen the better. Large specimens are more likely to be sexually mature and to have mature gonads. What sex is the specimen you selected? Do you need to observe the animal to know that?
If your wetmount is thin enough, increase the power stepwise until you are at 400X. You must be very careful using the high dry lens (40X) with wetmounts because of their thickness. If the objective lens touches the coverslip you will either have to remove some water to make the preparation thinner, or limit your observations to 200X. The former is preferable, if feasible.
Be careful that you do not allow the slide to dry out during your observations. Some drying may be beneficial because it will limit the movement of the animals and make them easier to observe but they will become inactive and contracted if drying is excessive. You will occasionally have to add a drop of water to the edge of the coverslip. It may be necessary that you make a second, or even a third, wetmount if your specimens do not remain active long enough for you to complete your observations.
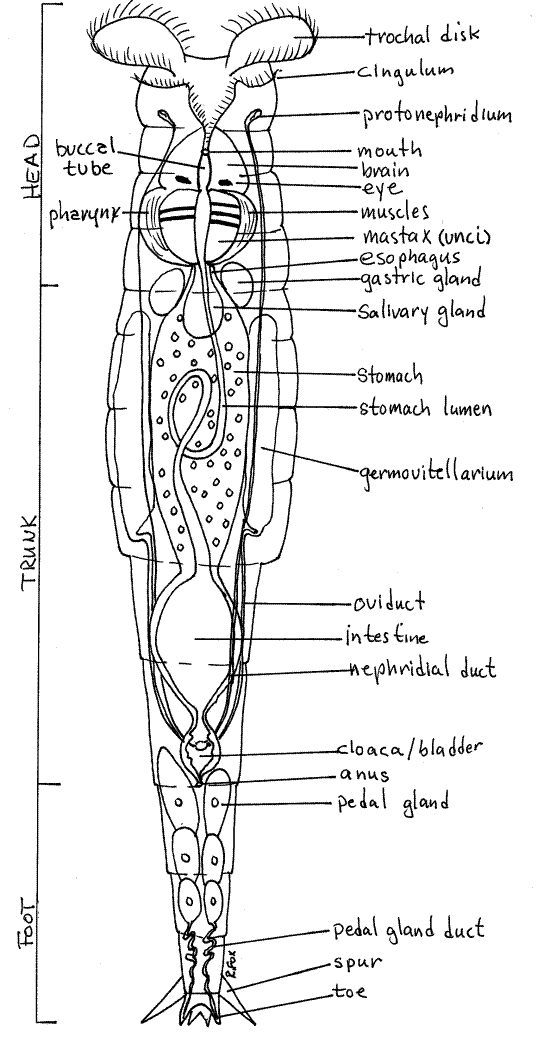
Rotifers are covered by an intraepidermal lorica, which may be thick or thin. The In bdelloids the lorica is in rings, or annuli, that telescope into each other to change the length of the worm (Fig 1, 23-17). Think about the muscles that would be required to accomplish this (Fig 23-13). You should be able to distinguish between the middle trunk, anterior head, and posteriorfoot. The trunk is the thick central region of the body whereas the head and the foot are narrower. Watch as the animal retracts, by telescoping, the head and/or foot into the trunk.
Find the ciliated corona at the anterior end of the head (Fig 1). Watch the cilia beat. The corona is used for swimming through the water and for creating a feeding current into the mouth. In some moss species the corona is small. A sensory dorsal antenna on the dorsal surface of the head can be seen only if the animal gives you a side view (Fig 23-14C).
The rotifer pharynx contains a unique mastax composed of several cuticular jaws used for manipulating food. The mastax can be seen near the junction of the head with the body. All bdelloids have a malleate mastax that has two large platelike unci that grind food particles (Fig 1). In an active rotifer you can watch the unci move against each other.
The posterior end of bdelloid rotifers bear small toes connected with pedal glands in the foot (Fig 1, 23-17). The glands secrete an adhesive which the rotifer uses to attach temporarily to the substratum. The toes are at the posterior end of the foot but they are difficult to see. Spurs, which look like toes, are easy to see and may be confused with toes. The spurs are dorsal and relatively large and are thus easy to see in a rotifer that is attached to the slide and has its dorsum facing you. The toes are ventral and smaller. They are hidden by the foot in a specimen with its back to you. You must watch until the animal presents you with a favorable angle and then be satisfied with a fleeting glimpse of the toes.
Some aspects of the internal anatomy may be visible. A large spherical urinary bladder may be evident at the junction of the foot and trunk (Fig 1, 23-8A,B). If your specimen is mature you can probably see two large germovitellaria, or combined ovaries and yolk producing organs, on the right and left sides of the trunk. Each may be dominated by a large yolky egg.
Figure 1 Ventral view of a female benthic rotifer, Philodina. Rotifer69La.gif
References
Pennak RW. 1989. Fresh-water invertebrates of the United States, 3 rd ed. Wiley, New York.
Ruppert EE, Fox RS, Barnes RB. 2004. Invertebrate Zoology, A functional evolutionary approach, 7 th ed. Brooks Cole Thomson, Belmont CA. 963 pp.
Ruttner-Kolisko A. 1074. Plankton rotifers biology and taxomomy. Binnengewasser supplement 36(1):1-146.
Wallace RL, Snell TW . 2001. Phylum Rotifera, pp 195-254 in Thorp JH, Covich AP (Eds), Ecology and classification of North American freshwater invertebrates, 2 nd ed. Academic Press, San Diego. 1056 pp.
Supplies
Dissecting microscope
Compound microscope
8-cm culture dish
Slides, coverslips