Invertebrate Anatomy OnLine
Asterias forbesi ©
Sea Star
25may2007
Copyright 2001 by
Richard Fox
Lander University
Preface
This is one of many exercises available from Invertebrate Anatomy OnLine , an Internet laboratory manual for courses in Invertebrate Zoology. Additional exercises, a glossary, and chapters on supplies and laboratory techniques are also available at this site. Terminology and phylogeny used in these exercises correspond to usage in the Invertebrate Zoology textbook by Ruppert, Fox, and Barnes (2004). Hyphenated figure callouts refer to figures in the textbook. Callouts that are not hyphenated refer to figures embedded in the exercise. The glossary includes terms from this textbook as well as the laboratory exercises.
Systematics
Echinodermata P, Eleutherozoa, Asteroidea C, Forcipulatida O, Asteriidae F, (Fig 9-26, 27-12, 28-62)
Echinodermata P
Echinoderms are secondarily radially symmetric deuterostomes whose ancestors were bilaterally symmetric. The adult radial symmetry is pentamerous with body parts occurring in fives or multiples thereof. Echinoderms have strong affinities with the ancestral trimeric deuterostomes especially in the tripartite organization of the coelomic cavities. Echinoderm larvae have the coelom divided into three regions, as is typical of the early coelomates, and these regions have important adult derivatives. All echinoderms are marine and benthic. About 6000 Recent species are known but the fossil record includes 13,000 extinct species.
An important echinoderm apomorphy is the water vascular system that in most groups functions in support of locomotory tube feet but is also important in gas exchange, excretion, and feeding. The body wall includes a thick connective tissue dermis in which calcareous ossicles (little bones) are almost always an important component. These ossicles make up an endoskeleton which assumes different forms in different taxa. In most echinoderms calcareous spines of various sizes and shapes arise from the dermis and extend from the body surface and are alluded to by the name echinoderm (= spiny skin). The connective tissue is mutable and its consistency is under nervous control.
Excretion in echinoderms is accomplished by simple diffusion of metabolic wastes (ammonia) across thin permeable regions of the body wall. A variety of gas exchange structures, including the tube feet, is found in various echinoderms. A hemal system is present but its role in transport is still poorly understood and the chief transport system is the circulating fluid of the various coelomic compartments. The hemal system may be through transport system that delivers nutrients from the gut to these compartments for local distribution. The nervous system consists of two central intraepidermal nerve rings from which arise radial nerves to the periphery. Echinoderms are gonochoric and fertilization is usually external.
Eleutherozoa
Eleutherozoans are mobile echinoderms in which the oral surface is oriented against the substratum. A madreporite and locomotory tube feet are present. Polian vesicles and Tiedemann’s bodies may be present on the ring canal. Movable spines are present. Eleutherozoa includes all Recent echinoderms except for its sister taxon, Crinoidea.
Asteroidea C
Asteroids are the sea stars, which are the best known echinoderms. Sea stars usually have five arms, but sometimes more, radiating from a central disk. The ossicles of the body wall are rodlike and articulate via fibrous junctions to form a flexible grid. Respiration is with the tube feet and papulae. Each arm has an eyespot at its tip. A pair of large pyloric ceca and a pair of gonads are present in each arm. Gastric hemal tufts are present. About 1500 Recent species are known.
Laboratory Specimens
The sea star, Asterias forbesi , is common in shallow water along the Atlantic Coast of North America from the Gulf of Maine to the Gulf of Mexico. Study a living specimen of this or a similar species if possible. If not, a preserved specimen can be used. Many exercises and demonstrations are possible with living specimens that are cannot be accomplished with preserved stars. The specimen should be in a dissecting pan of seawater or isotonic magnesium chloride if living, or tapwater if preserved. A dissecting microscope will be needed for some of the exercise.
External Anatomy
The body is divided into a central disk from which radiate five arms (Fig 1, 28-13). The principal body axis, and the axis of symmetry, is the short oral-aboral axis, which passes vertically through the center of the disk. The animal's pale lower side is the oral surface and structures on this side are said to be oral. The dark upper side is the aboral surface and structures on this side are aboral. Structures remote from the axis are said to be peripheral whereas those near the axis are central. In radially symmetrical animals anterior-posterior, dorsal-ventral, right-left are irrelevant and have no meaning.
Aboral Surface
Find the calcareous, orange madreporite on the aboral surface of the disk (Fig 1). Examine it with the high power of the dissecting microscope and note its grooved surface. Numerous microscopic pores in the bottoms of the grooves open into canals (stone canal and axial canal) of the internal water vascular system (Fig 28-15).
>1a. If you have a living specimen, place a tiny drop of carmine/seawater or chalk dust and seawater on the surface of the madreporite and observe it with the dissecting microscope. Look for evidence of currents across the surface of the madreporite. Such currents keep the surface free of debris. <
Orient the star with the aboral side up and with the madreporite close to you. The arm on the left of the madreporite is arm I, arm II is to the right of the madreporite, and the remaining arms are numbered sequentially moving counterclockwise around the star (Fig 1). A radial axis passing from the center of the disk outward along the midline of any arm is a radius, orambulacral axis, of which there are five. Any axis bisecting the angle between any two adjacent arms is an interambulacral axis, or interradial axis, and there are five of these also. An interambulacral axis passes through the madreporite.
Figure 1 Aboral view of Asterias. Asteroid13La.gif
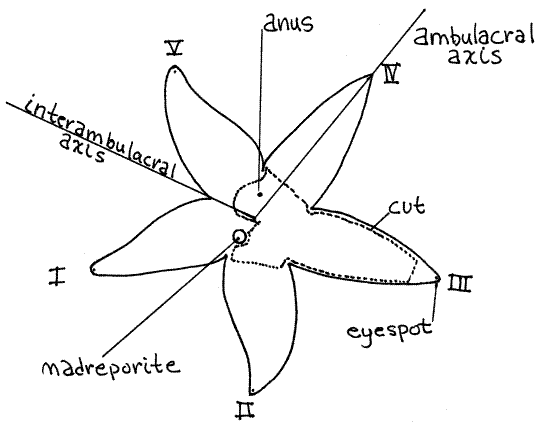
If necessary, remove the congealed mucus and other debris from the aboral surface of the disk with strong jets of water from a squeeze bottle or Pasteur pipet before examining it with the dissecting microscope. The surface is covered by a monociliated epidermis but this will not be apparent to you although the activity of the cilia can be demonstrated with living specimens.
>1b. If your specimen is living, place a drop of carmine-seawater suspension on a horizontal region of its aboral surface and observe the motion of the carmine particles with magnification. <
On the aboral surface notice the numerous small fixed spines, so-called because they are fixed in position and cannot move. These spines are extensions of the calcareous endoskeleton in the body wall. Gently push one of the spines with the tip of a microneedle to see if it moves. Look closely at the spines with the highest magnification of the dissecting microscope and confirm that they are indeed internal and are covered by a thin layer of living tissue, the epidermis. Each spine is surrounded by a circle of short-stemmed, white pedicellariae(singular: pedicellaria, Fig 28-11A). Pedicellariae have an endoskeleton of ossicles (Fig 28-10).
>1c. Remove several pedicellariae with your fine forceps and place them in a drop of bleach on a microscope slide. Wait a few minutes for the organic tissue to be oxidized and then place a coverslip over the drop. Examine it with the compound microscope and look for the jaw-like ossicles. These pedicellariae contain three ossicles. One is a short basal piece in the stalk of the pedicellaria whereas the other two support the two jaws (Fig 28-10). Tiny muscles extend between these ossicles to operate the jaws but these will have been removed by the bleach. Examine an ossicle with 400X to see the numerous pores that perforate it. If there is too much soft tissue remaining, the pores, or even the ossicles themselves, may not be visible. Try looking at several ossicles with carefully adjusted light if necessary to find pores. Such pores are characteristic of echinoderm ossicles and prevent the spread of cracks. <
Between the spines are many soft, thin-walled, translucent, fingerlike papulae (Fig 2, 28-14, 28-15). Papulae are thin-walled diverticula of the coelom through the body wall and are its respiratory organs. The ciliated peritoneum generates a bidirectional flow of fluid into and out of the papulae. The papulae are muscular and can be retracted into the surface of the body wall. They may be retracted and inconspicuous in preserved specimens. If you have a living specimen touch a papula with the microneedle and observe its response.
>1d. Observe a papula of a living specimen closely with high magnification of the dissecting microscope and look for circulating coelomocytes in the coelomic fluid inside it. Note that the flow is bidirectional. What causes the motion of the coelomocytes? Touch a papula with a nadel and note its response. <
>1e. Inject carmine/seawater suspension into the coelom of a living animal (not the one you plan to dissect) and observe the papulae with your highest magnification. Look for circulating carmine particles in the papulae. Examine the animal occasionally for the remainder of the period and note changes in the appearance of carmine in the papulae. Carmine particles will gradually accumulate in the tips of the papulae which eventually pinch off, thus ridding the coelom of this foreign material. <
The anus is located near the center of the aboral surface but is almost impossible to demonstrate externally. It is surrounded by a palisade of tiny ossicles, much smaller than the spines that stud the surface of the disk and is in an area free of papulae.
Oral Surface
Turn the animal over and study the oral surface. Find the large mouth in the center of the disk, surrounded by the thin peristomial membrane (Fig 28-6B). The yellowish-orange curtain-like folds of the cardiac stomach may be visible inside the mouth.
Five deep ambulacral grooves radiate outward from the mouth, one along the midline of the oral surface of each arm (Fig 3, 28-6B). Each groove lies on an ambulacral axis. The numerous soft, tubular structures projecting into the groove from either side are the tube feet, or podia. Two rows of tube feet are present on each side of the groove. The tube feet of Asteriasbear suckers at their distal ends (Fig 3, 28-12B). Note the rows of long, flattened movable spines on each side of the ambulacral groove (Fig 3). The word ambulacrum is Latin for "covered way," an apt name as these spines are used to cover the groove to protect the tube feet.
>1f. Push a tube foot of a living star with a microneedle and observe the response of the foot and of the movable spines. Is the name “ambulacrum” appropriate? <
Look at the tip of one of the arms (Fig 28-6A). As is usual in radially symmetrical animals, the sensory structures are arrayed around the periphery, which in sea stars is the tips of the arms. Several long, narrow sensory tube feet extend from the tip of each arm. These are easily seen in living specimens but contract and become inconspicuous in preserved material. They have chemo- and mechanoreceptors. At the tip of the arm is a small circle of short, blunt movable spines that are not associated with pedicellariae. These spines surround a small, pale red or yellow eyespot (Fig 1, 28-6A). The eyespot is on the oral surface of the arm, almost at the tip.
>1g. While the star (if living) is on its aboral surface, it will probably attempt to right itself, or turn back over onto its oral surface employing a series of movements known as the "righting response". Watch the process from start to finish and record your observations (Fig 28-13). What is the first step? Are the tube feet involved? How many arms are involved? In what order? Do you think there are muscles in the body wall? You could test the importance of tube feet by placing the star on a sand bottom and noting its effect on righting ability. <
Internal Anatomy
If you have a living specimen, replace the water in the dissecting pan with isotonic magnesium chloride at this time. Preserved specimens should remain in tapwater.
" Use a robust pair of scissors to cut the end from arm III about 2 cm from its tip as indicated in Figure 1. Insert the sharp point of the scissors into the opening and cut along the side of the arm until you reach the disk as indicated by the dotted lines on the figure. Make a second cut, similar to the first, on the other side of the same arm. Do not lift the aboral body wall off the disk yet. Extend the cuts around the margin of the disk and across the bases of the other arms but DO NOT cut between the madreporite and the edge of the disk. Do not damage the madreporite or structures lying inside the body wall.
Gently lift the aboral body wall slightly and with a blunt probe or teasing needle carefully free it from the underlying tissues to which it is connected by mesenteries. Do this without damaging the soft tissues. Lift the body wall of the disk enough to see beneath it and look on its inside surface to find the point at which the inconspicuous intestine enters it to reach the anus. The small, lobed, olive-green (in life) rectal cecum surrounds the intestine and obscures its junction with the body wall (Fig 2, 28-16).
Figure 2. Asterias in aboral view with the body wall removed from the disk and two arms. The pyloric ceca have been removed from arm V. asteroid14La.gif
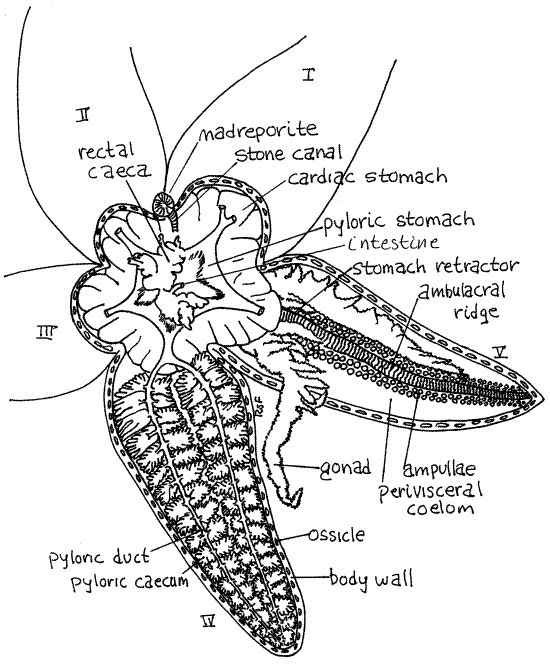
Once you have found the cecum, free it from the body wall so it remains with the rest of the viscera. Cut across the aboral disk so the madreporite remains intact (Fig 1). Remove the now free portions of the body wall. The intestine will probably be destroyed by this procedure. Leave the organs of the body cavity intact. Set the body wall aside but keep it immersed.
Make a preliminary examination of the body cavity and its organs. Identify the major organs now so you can use them as landmarks later. The space you have exposed is theperivisceral coelom (Figs 2, 3, 28-15). Most of the interior of the central disk is occupied by the cardiac stomach. It is a large mass of thin orangish tissue. It is highly extensible and can accommodate large prey when extended outside the body. Two large, brownish, greenish, or creamy-white (in life) pyloric ceca (= digestive ceca, hepatic ceca, digestive glands) occupy most of the aboral half of the arms (Fig 2, 3 28-16A).
Two gonads lie in the oral half of the each arm hidden by the pyloric ceca (Fig 2, 3, 28-16A). Their size depends on reproductive condition and they may be very small or absent in immature or reproductively inactive specimens.
Lift the pyloric ceca and gonads to reveal the floor of the arm. Locate the conspicuous, raised ambulacral ridge running lengthwise along the middle of the arm (Figs 2, 3, 28-16A,C). It is the internal manifestation of the ambulacral groove you saw on the outside of the arm. It is formed of sequentially arranged ambulacral ossicles in the body wall. The divisions between adjacent ossicles are clearly visible grooves that give the ridge a distinctly striated appearance.
On either side of the ridge find the double row of bulbous ampullae of the tube feet of the water vascular system (Fig 2, 3, 28-15). These protrude into the perivisceral coelom and are covered by its peritoneum.
Body Wall
Examine the cut edge of a part of the body wall using moderate magnification of the dissecting microscope. It consists of a thin, outer, ciliated epidermis, a thick, easily-seen connective tissue dermis, and thin, inner peritoneum (Fig 3, 28-15). Look at the dermis. It contains collagen fibers and many calcareous dermal ossicles, or "little bones", which may have been crushed by the scissors. Note that some of the ossicles bear spines.
>1h. Place a piece of the excised body wall in a 6-cm culture dish and cover it with bleach. Inspect it occasionally and transfer it to tapwater when enough of the soft tissue has been oxidized to reveal the endoskeleton. Do not leave it in the bleach longer than necessary to expose the ossicles. Test a small piece of the endoskeleton with 8% HCl to determine if it is siliceous or calcareous. <
Coelom
The echinoderm coelom has many subdivisions but only the perivisceral coelom and water vascular system will be studied in this exercise. The perivisceral coelom is the largest of the coelomic compartments and is the chief body cavity. Most of the space in the arms and disk is perivisceral coelom (Figs 2, 3) and the viscera are located in it. The perivisceral coelom is derived from the right and left metacoels of the larva (Fig 28-4).
Study the inner surface of the aboral wall of the arm which you removed earlier and set aside. It is covered inside by a thin, transparent, ciliated epithelium, which is the peritoneum of the perivisceral coelom. Activity of its cilia circulates coelomic fluid to distribute food and oxygen to the surrounding tissues.
>1i. Place a drop or two of carmine-seawater suspension on a horizontal part of the body wall of your dissected living specimen and watch for motion of the particles. The movement is due to peritoneal cilia. <
Figure 3. Cross section of an arm of Asterias. Asteroid15Lb.gif
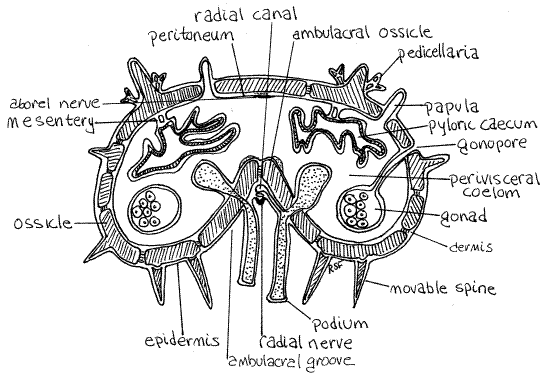
Note the numerous small pores in the body wall and that the peritoneum extends into them. These are openings into clusters of papulae (Fig 3, 28-14). Using magnification and good light, look straight into one of the pores and you will see that it opens into several papulae.
>1j. If you are dissecting a living star, place some carmine particles on the peritoneum in the vicinity of one of these pores and watch particles enter one side of the opening and come back out the other side. Is this consistent with your earlier observations of bidirectional flow in papulae? <
Many of the organs of the perivisceral coelom, such as the pyloric ceca and gonads, are suspended from the body wall by mesenteries (Fig 3). The mesenteries were necessarily destroyed when you removed the aboral body wall.
Digestive System
The short gut extends vertically from the mouth in the center of the oral disk to the anus near the center of the aboral disk. It consists, in order, of mouth, esophagus, cardiac stomach, pyloric stomach (with pyloric ceca), intestine (with intestinal ceca), and anus. It is lined internally with a ciliated epithelium and is surrounded by the perivisceral coelom.
The mouth opens into a short indistinct esophagus which you will not see at present (Fig 28-15). The esophagus opens into the large, thin-walled, orange cardiac stomach filling most of the perivisceral coelom of the disk (Fig 3, 28-15, 28-16A). When feeding, Asterias everts the cardiac stomach from the mouth to surround its prey. Digestion begins extracellularly in the cardiac stomach while the prey, and stomach, are still outside the mouth. Partially hydrolyzed materials are moved to the pyloric stomach by ciliary currents. From here they enter the hollow pyloric ceca where both extracellular and intracellular digestion take place. The products of digestion can be stored in the cells of the pyloric ceca or, presumably, diffused into the surrounding perivisceral coelom.
Five pairs of so-called stomach retractor muscles anchor the cardiac stomach to the sides of the ambulacral ridges (Fig 2). There is little muscle in them, however, and, as they are primarily connective tissue, they probably are more important as anchors than as retractors.
The cardiac stomach opens at its aboral end into the much smaller pyloric stomach (Fig 2, 28-16A, 28-15). The pentagonal outline of the pyloric stomach makes it easy to recognize atop the cardiac stomach.
The ten large pyloric ceca are hollow diverticula from the pyloric stomach and the two ceca of each arm share a common connection with the margin of the pyloric stomach (Fig 2, 28-16A, 28-15). Each cecum extends most of the length of its arm and consists of a long pyloric duct with numerous branches (Fig 2). Tiny food particles are phagocytized by the cecal epithelium and digested intracellularly.
" Cut or tear one of the large branches of a pyloric cecum to show yourself that it is hollow and has relatively thick walls. Its thick, endodermal epithelium is secretory and absorptive.
The tiny, inconspicuous intestine extends aborally from the center of the pyloric stomach to the anus (Fig 2, 28-16). The lobed intestinal cecum is attached to the intestine. It, and the intestine, may have been destroyed by the removal of the aboral disc.
" Open the cardiac stomach and look inside. Push the billowy folds of the cardiac stomach aside and trace the gut orally to the mouth. The short and indistinct region between the cardiac stomach and the mouth is the esophagus.
" Remove the gut from the animal to reveal the region around the mouth. This will necessitate cutting the pyloric duct and the two stomach retractor muscles in each arm. Cut the connection between the esophagus and peristomial membrane, taking note of the membrane as you may not have seen it earlier.
Water Vascular System
Removal of the gut reveals most of the central features of the water vascular system (Fig 2, 3, 4, 28-12A). Gently deflect the part of the aboral disk containing the madreporite and look below it for the stone canal. This curved duct extends orally from the madreporite and has calcareous skeletal rings for support. Because it is calcified, it is firm to the touch.
An obscure vertical partition, the interbrachial septum, is located between the bases of each pair of adjacent arms. Thus, the star has five interbrachial septa. The stone canal is in the interbrachial septum between arms I and II (Fig 4). Also in this septum is the soft axial complex, which surrounds the stone canal but in gross dissection appears to be beside it. The axial complex is composed of the axial gland of the hemal system and the axial canal of the coelomic system (Fig 28-15). It may be difficult to see in preserved specimens.
Figure 4. Aboral view of the mouth frame of Asterias. Asteroid16La.gif
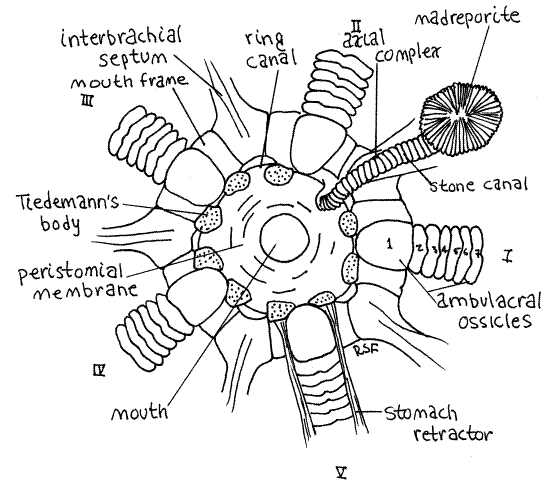
Trace the stone canal orally and note that it crosses a heavy, white, circular or pentagonal, skeletal ring known as the mouth frame (Fig 4). The stone canal extends to the inner surface of the mouth frame where it joins the inconspicuous (very) ring canal (= water ring) of the water vascular system. The thin, membranous walls of the ring canal are not calcified and they adhere closely to the inner curve of the mouth frame and cannot be distinguished from it. Its position, however, is marked by nine small, soft, low, spongy Tiedemann's bodies on the inner margin of the frame (Fig 4). These organs are evaginations of the ring canal. Typically 10 such bodies are present, two associated with each interbrachial septum, but in Asterias one is missing where the stone canal joins the ring canal so only nine are present. The lumina of these bodies are continuous with the ring canal and it is thought that they remove foreign particles, by phagocytosis, from the circulating fluid of the water vascular system.
Asterias has no Polian vesicles although these are present in many asteroids (Fig 28-12A). You may see the ten isolated ampullae of the buccal tube feet on the aboral surface of the mouth frame. They pass through pores in the frame to connect with special enlarged buccal tube feet around the mouth.
The ring canal gives off five radial canals, one for each arm (Fig 3, 28-12A). These canals leave the outside surface of the ring canal and pass along the ambulacral groove outside the ambulacral ossicles of the skeleton (Fig 2) but they are difficult to see.
Single, unpaired lateral canals arise, alternately to the right and left, from the radial canals and extend to the podial complices, one lateral canal to each (Fig 3). Like the radial canals, the lateral canals are difficult to demonstrate in gross dissection. Each podial complex consists of tube foot (= podium) and its ampulla.
You have already seen the tube feet in the ambulacral grooves on the oral surfaces of the arms. The aboral end of each tube foot narrows, penetrates the overlying ambulacral ossicle and is continuous with an ampulla which bulges into the perivisceral coelom (Figs 2, 3). Like everything else in the perivisceral coelom, the ampullae are covered by monociliated peritoneum. Their lumina are also lined by peritoneum since the space inside is a coelomic cavity, i.e. the water vascular system.
Use a microneedle to push an ampulla aside so you can see that it narrows orally and becomes a slender tube penetrating the ambulacral ridge. Carefully insert the needle into the ampulla and pass it through the pore in the body wall to emerge on the other side in the middle of a tube foot.
>1k. In a living star, tear or cut one of the ampullae so you can place a little carmine-seawater inside it. The internal peritoneum is ciliated and you should see evidence of ciliary activity.<
Asteroids are said to have an open ambulacral system because the radial canal, lateral canals, and radial nerve are on the outside of the body wall skeleton (Fig 3).
" Make a fresh cut across the tip of an arm and look carefully at the cut surface of the tip with magnification. Examine the midline, in the ambulacral groove, between the two double rows of tube feet. Try to discern the inconspicuous radial nerve (Fig 3). The nervous system is epithelial so the nerves are part of the epidermis. The radial nerve is a thickened area of epidermis that runs the length of the arm between the tube feet.
Above the nerve is a very small radial hemal vessel and its surrounding coelomic space (hyponeural coelom). The vessel will not be seen in gross dissection but you may see the coelom. The radial water canal is above the blood vessel lying between the superficial body wall muscles and the ossicles, immediately oral to the ossicles.
>1l. Asterias is negatively geotactic and tends to move up on vertical surfaces such as the walls of aquaria. If seastars are kept in aquaria in your laboratory, observe them periodically for the next few days and note their location in the tank. Where do you usually find them? Is this observation consistent with being negatively geotactic? <
Reproductive System
Asteroids are gonochoric and fertilization is external. Each individual has a pair of gonads in each arm (Figs 2, 3, 28-12B, 28-16A). The gonads may be very large if the individual is sexually mature or, if the specimen is immature or reproductively inactive, they may be so small as to be difficult to find. If they are small, they will be located on the oral surface of the base of each side of each arm. Every gonad connects to its own gonopore via an inconspicuous gonoduct. The tiny gonopores are on each side of the base of the arm, on its aboral surface.
HemalSystem
The hemal system of asteroids is difficult to demonstrate in gross dissection. The hemal system remains the least understood of echinoderm organ systems. It is a blood vascular system in that it is a space in connective tissue. It is atypical in that the blood vessels end blindly and there is no continuous circulation of blood around a circuit. Blood vessels extend to the pyloric ceca, gonads, nervous system, water vascular system, and perivisceral coelom.
Excretory System
To date there has been no demonstration of a special osmoregulatory or excretory systems in echinoderms. Coelomic and interstitial fluids are osmotically similar to seawater. Leakage of water vascular system fluid across the pressurized tube feet is countered by the slightly higher osmolarity of water vascular system fluid. The end product of nitrogen metabolism is ammonia, which in asteroids is eliminated by diffusion from the papulae and tube feet.
Respiratory System
In echinoderms the hemal system does not distribute oxygen to the tissues. Instead, most major coelomic spaces are associated, at least indirectly, with respiratory surfaces and gasses are transported by the circulating coelomic fluid. The papulae and tube feet are the respiratory structures for the perivisceral coelom and the tube feet serve this purpose for the water vascular system.
Nervous System
Little, if any, of the nervous system is apparent in gross dissection. It is intraepithelial and consists of a plexus extending through the body wall with concentrations forming a circumoral ring from which arise radial nerves into each arm.
The five radial nerves are thickened bands of epidermis lying on the ambulacral axis of the oral surface of each arm (Fig 3, 28-15). They may sometimes be seen by pushing aside the rows of tube feet. Each of the nerves is a thin, slightly raised, brown or yellow line extending from the nerve ring to the eyespot at the tip of the arm. If a radial nerve is followed to the disk it can sometimes be seen to join the circumoral nerve ring around the mouth.
Arm Cross Section Slide
Examine a commercially prepared slide of a cross section of an arm with the compound microscope at with properly adjusted light and sharp focus (Fig 3, 28-12B). Use 40X for most of your observations occasionally switching to 100X and 400X when needed. These tissues have been decalcified to permit sectioning, so ossicles are not present. Further, because the specimens have to be small enough to fit on a slide, they are not mature and do not have gonads.
Orient the slide so the aboral surface is up when viewed through the microscope (tube feet are oral, pyloric glands are aboral). Find the thick body wall enclosing the spaciousperivisceral coelom. The three layers of the body wall are the outer epidermis, a thin but conspicuous inner mesothelium (peritoneum), and a thick middle connective tissue dermis. Projections from the surface of the body wall are spines, pedicellariae, or papulae. Spines and pedicellariae normally contain calcareous dermal ossicles but these are absent in these preparations making the structures difficult to identify. The decalcified remains of the ossicles, however, are present and visible. In general pedicellariae will appear with forked tips representing their jaws whereas spines do not have double tips. Papulae, if present, are hollow evaginations of mesothelium that protrude through the body wall and should appear as such (Fig 28-14) although the plane of section usually does not pass through the connection with the perivisceral coerlom.
The two large lobed structures in the perivisceral coelom are the pyloric glands (= digestive ceca). These are diverticula of the pyloric stomach and are the sites of enzyme secretion, absorption, phagocytosis, and intracellular digestion. Note the thick endodermal lining of the hollow ceca. Each cecum is suspended from the aboral body wall by a mesentery, which is a double layer of mesothelium.
On the aboral midline of the arm, the mesothelium is thickened to form an aboral radial nerve (Fig 3, 28-12B). This is a motor nerve. Immediately aboral to the nerve is a small radial aboral muscle with longitudinal fibers.
The ambulacral ridge arches aborally into the perivisceral coelom. It is a feature of the oral body wall of the arm. It is the inner manifestation of the ambulacral groove visible outside, on the oral surface of the arm. Study this area carefully keeping in mind that these slides are sections, not wholemounts, so you are not likely to see perfect views of structures such as tube feet or lateral canals. Most conspicuous are the tube feet (podia), of which most slides will have four, or parts of four, depending on the location of the plane of section. The tube feet are in the ambulacral groove. The section may or may not include a sucker at the end of the tube foot. The slide may or may not include ampullae extending from the tube feet into the perivisceral coelom. The coelom of the ampulla is separated from the perivisceral coelom by a double layer of monociliated mesothelium. Even if ampullae are present in your section, their connection with the tube feet may not have been captured.
The radial canal (of the water vascular system) is a faintly visible more or less oval ring on the oral midline of the arm. Again, depending on the plane of section, you may or may not see a lateral canal extending from the radial canal to a tube foot. Sometimes only a portion of a lateral canal is present extending from either, or both, the radial canal and tube foot but not connecting. In perfect sections you will see not only the complete lateral canal but its one way valve as well.
The epidermis on the oral midline of the arm is thickened to form a radial nerve, which is mostly ectoneural. Immediately aboral to the radial nerve and oral to the radial canal, is a large space which is mostly the hyponeural radial canal (Fig 28-12B, 28-14). The hyponeural radial canal is a coelomic space surrounding the hyponeural radial hemal vessel although you are not likely to see the latter. Part of the mesothelium of the hyponeural radial canal contributes hyponeural (mesothelial) neurons to the radial nerve.
A pair of radial oral muscles can be seen on either side of the ambulacral ridge just outside the mesothelium of the perivisceral coelom (Fig 3).
Starfish Development
Slides of early starfish developmental stages may be available in the laboratory. These may includ all early developmental stages on a single side or on separate slides. The stages are usually stained with colors that have no meaning. Many of the stages are spheres which must be distinguished from each other using various clues. Keep in mind as you interpret the embryos that they are wholemounts, not sections, and their appearance will vary depending on the level of your plane of focus (optical section).
First in the developmental series is the unfertilized female gamete, the egg, or ovum (Fig 5A). It is a large sphere recognized by the presence of a conspicuous female pronucleus (= germinal vesicle) which contains a distinct nucleolus. The plasma membrane is the outer boundary of the cell and, since the egg has not been fertilized, there is no fertilization membrane.
Figure 5. Starfish early development. A. Ovum, B. Zygote, C. 2-celled embryo, D. Polar view of 4-celled embryo, E. Polar view of 8-celled embryo, F. Early blastula, G. Late blastula, H. Early gastrula. Asteroid63L.gif
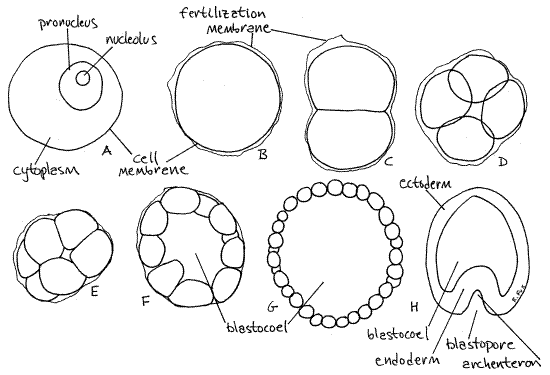
Once fertilized the egg becomes a zygote (Fig 5B). This stage is also a sphere but a wrinkled, transparent fertilization membrane is present around the cell and the pronucleus can no longer be seen. The fertilization membrane is produced immediately after fertilization and prevents penetration by additional sperm. It will persist until the blastula stage. The zygote, which is also a single cell, is the same size as the ovum.
Search the side for examples early cleavage stages, namely 2-cell, 4-cell, and 8-cell embryos, each enclosed in a fertilization membrane (Fig 5C, D, E). Pay attention to the orientation of the blastomeres with respect to each other, especially those of the 8-cell stage. Observe an 8-cell embryo and verify that the cell arrangement is typical of radially cleaving embryos. Is this consistent with what you expect of deuterostome embryos? _________ Notice the relative size of the embryos and their blastomeres. One of the tasks accomplished by early development is conversion of the enormous ovum to a multitude of much smaller cells, closer in size to normal somatic cells. The embryo does not grow during this period so, since cells are dividing, they must be getting smaller.
Subsequent divisions produce ever smaller cells which are arrayed in a hollow ball known as a blastula, specifically a coeloblastula (Fig 5F, G). Is this the type of blastula expected of deuterostome embryos? ________ Is the fertilization membrane still present? _________ The blastula is ciliated and if these embryos were alive you could see them rotating inside their fertilization membranes. Several ages of blastulae will be present. The youngest will have about 32 large, recognizable cells. You can see the individual cells forming the blastula wall. Focus up and down through the sphere to reveal the hollow interior of the ball. The interior space is, of course, the blastocoel.
As divisions continue the cells in the blastula wall get smaller and smaller until they can no longer be distinguished from each other. You can, however, still see the blastocoel in the center and the wall of cells enclosing it. Note that the embryo is still about the same size as the original ovum but its cells are much smaller.
Eventually one end of the blastula thickens in preparation for gastrulation. This end then invaginates to form a double walled embryo known as a gastrula (Fig 5H). Is invagination the gastrulation process you expect of deuterostomes? _________ The early gastrula has only a short invagination but it will rapidly increase in size and you should be able to find examples of several ages. The blastocoel remains but there is now a second cavity, the archenteron, or embryonic gut. The archenteron opens to the exterior by an opening, the blastopore. The outer wall of the gastrula is ectoderm whereas the wall of the archenteron is endoderm. Eventually pouches of the archenteron will form (by enterocoely) the coeloms and mesoderm. Is this the way you expect mesoderm and the coelom to form in deuterostomes? _________
The asteroid gastrula develops into a series of two larvae, the first of which is the bipinnaria (Fig 6, 28-3) followed by the brachiolaria larvae. The larvae are ciliated and feed on diatoms in the plankton (planktotrophic). Once the embryo begins feeding it can start to grow. The larvae are bilaterally symmetrical.
Several examples of bipinnaria larvae should be present on the slide. The bipinnaria has one or two locomotor ciliary bands. Young larvae have only one band but older larvae have two. (Some authors consider the younger larva to be a dipleurula larva). Find a larva in side view, either right or left, such as the left side view in Figures 6A and 28-18A. A side view is the easiest to interpret although it does not reveal the bilateral symmetry of the embryo (no more than looking at you from the side would give a hint of your symmetry). The blastopore has become the anus and a new opening appears and becomes the mouth. Is this as expected of deuterostome embryos? _________ To what does the word deuterostome refer anyway? __________________________________
The most conspicuous feature of the larva is its C-shaped gut. The ventral border of the larva is concave and the mouth is here, on the ventral midline near the middle of the ventral surface. The esophagus extends dorsally from the mouth and then curves posteriorly to join the stomach. The thick stomach wall is usually the most intensely stained part of the embryo. Theintestine exits the stomach and extends to the anus. The embryo swims using a locomotor ciliary band that curves over the surface of the body enclosing the mouth but excluding the anus in the bipinnaria (Fig 6). As the early embryo ages, the anterior ventral lobe of the ciliary band separates to form two separate loops, the small anterior preoral loop and the larger anal loop that passes ventrally anterior to the anus (Fig 28-18A, C).
Figure 6. An early bipinnaria larva with a single ciliary band. A. Viewed from the left, B. Viewed dorsally. Asteroid62L.gif
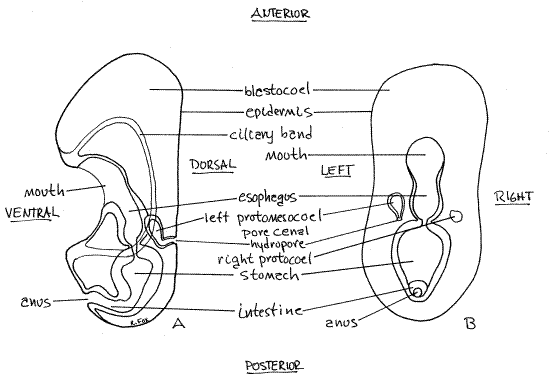
Be sure you understand the orientation of this larva and can recognize anterior, posterior, dorsal, and ventral. Are you looking at the right or left side? _______
Now that you understand the anatomy and orientation of a bipinnaria larva, scan the slide for a good example of one in either dorsal or ventral view (they will look the same). Either will reveal the bilateral symmetry characteristic of echinoderm larvae. In this view (Fig 6B, 28-18C) the mouth is in the approximate middle of the body and the anus is posterior to it. The gut, which is C-shaped (but not in this view), extends dorsally then posteriorly and then ventrally to connect mouth with anus. The locomotor band borders the embryo and pass immediately anterior to the mouth on the ventral surface but it is usually difficult to see.
On the left side of the embryo (but visible from either side of these transparent creatures) at about the level of the esophagus-stomach junction is a small pouch connected to the dorsal surface by a canal and pore (Fig 6, 28-4). The pouch is the left protomesocoel (= axohydrocoel, = coelomic vesicle), the canal is the pore canal, and the pore is the hydropore, which is the larval nephridiopore. During metamorphosis, the left protomesocoel becomes the water vascular system, the pore canal becomes the stone canal, and the hydropore is the presumptive madreporite. On some slides the right protocoel (dorsal sac) (Fig 6, 28-4) can be seen on the right, at the same level as the left, but smaller than the protomesocoel. It will become the pericardial sac around the heart.
With age the bipinnaria becomes a more advanced larva known as the brachiolaria. These larvae are not represented on the composite slides but the laboratory may have other slides of them available. The brachiolaria has five pairs of ciliated (for swimming) larval arms (brach = arm) (Fig 28-18B). The preoral loop of the ciliary band extends onto one anterior pair of arms whereas the other four pairs bear portions of the main ciliary band. The profusion of arms provides the surface area for the locomotor cilia.
Like the dipleurula and bipinnaria, the brachiolaria is bilaterally symmetrical. It is the brachiolaria that will settle out of the plankton onto a suitable substratum and undergo the metamorphosis in which it forsakes its ancestral bilateral symmetry in favor of secondary pentaradial symmetry (Fig 28-19, 28-4)
References
Balser E J, Ruppert EE . 1990. Structure, ultrastructure and function of the heart-kidney complex of Saccoglossus kowalevskii (Hemichordata: Enteropneusta). Acta Zool. 71:235-249.
Brooks WK. 1890. Handbook of Invertebrate Zoology. Bradlee Whidden, Boston. 352 p.
Brusca GJ. 1975. General patterns of invertebrate development. Mad River Press, Eureka, CA. 134 pp.
Bullough WS. 1958. Practical Invertebrate Anatomy (2nd ed). MacMillan, London. 483p.
Chadwick HS. 1923. Asterias. Liverpool Mar. Biol. Comm. Mem. 25:1-63, pls. 1-9.
Chia F-S, Koss R. 1994. Asteroidea, pp169-245 in Harrison FW, Chia F-S. (eds). Microscopic anatomy of invertebrates, vol 14. Echinodermata. Wiley-Liss, New York. 510pp.
Hyman LH. 1955. The Invertebrates: Echinodermata, vol. IV. McGraw-Hill, New York. 763pp.
Kume M, Dan K. 1957 (1968 translation from the Japanese). Invertebrate embryology. National Library of Medicine, US Public Health Service, Washington, DC. 605 pp.
Lawrence J. 1987. A Functional Biology of Echinoderms. Johns Hopkins, Baltimore. 340p.
Reid WM . 1950. Asterias forbesi, pp 515-523 in Brown FA. (ed) Selected Invertebrate Types. Wiley, New York. 597p.
Ruppert EE, Fox RS, Barnes RB. 2004. Invertebrate Zoology, A functional evolutionary approach, 7 th ed. Brooks Cole Thomson, Belmont CA. 963 pp.
Supplies
Living or preserved sea stars, preferably Asterias
Dissecting microscope
Compound microscope
Dissecting pan, about 20 x 30 cm
Isotonic magnesium chloride for living specimens
Seawater for living specimens
Pasteur pipet
Dissecting set with microdissecting tools
Longitudinal section slides of tube foot
Carmine seawater suspension (living specimens only)
Chalk dust (living specimens only)
Squirt bottle of seawater or tapwater
8% hydrochloric acid
6-cm culture dish
Dropper bottle of bleach
Hypodermic syringe and needle (living specimens only)
Sources
|
Item |
Source |
|
Living Asterias |
Woods Hole MBL |
|
Preserved Asterias |
Carolina, Wards |
|
Arm cross section slides |
Carolina, Wards, Triarch |
|
Starfish development slides |
Wards, Triarch, Carolina |
|
Brachiolaria larva slides |
Triarch |