Invertebrate Anatomy OnLine
Aplysia brasiliana ©
Sea Hare
3jul2006
Copyright 2001 by
Richard Fox
Lander University
Preface
This is one of many exercises available from Invertebrate Anatomy OnLine , an Internet laboratory manual for courses in Invertebrate Zoology. Additional exercises can be accessed by clicking on the links in the column on the left. A glossary and chapters on supplies and laboratory techniques are also available. Terminology and phylogeny used in these exercises correspond to usage in the Invertebrate Zoology textbook by Ruppert, Fox, and Barnes (2004). Hyphenated figure callouts refer to figures in the textbook. Callouts that are not hyphenated refer to figures embedded in the exercise. The glossary includes terms from this textbook as well as the laboratory exercises.
Systematics
Mollusca P, Eumollusca, Conchifera, Ganglionura, Rhacopoda, Gastropoda C, Euthyneura, Opisthobranchia sC, Anaspidea O, Aplysiidae F
(Fig 12-125)
Mollusca P
Mollusca, the second largest metazoan taxon, consists of Aplacophora, Polyplacophora, Monoplacophora, Gastropoda, Cephalopoda, Bivalvia, and Scaphopoda. The typical mollusc has a calcareous shell, muscular foot, head with mouth and sense organs, and a visceral mass containing most of the gut, the heart, gonads, and kidney. Dorsally the body wall is the mantle and a fold of this body wall forms and encloses that all important molluscan chamber, the mantle cavity. The mantle cavity is filled with water or air and in it are located the gill(s), anus, nephridiopore(s) and gonopore(s). The coelom is reduced to small spaces including the pericardial cavity containing the heart and the gonocoel containing the gonad.
The well-developed hemal system consists of the heart and vessels leading to a spacious hemocoel in which most of the viscera are located. The kidneys are large metanephridia. The central nervous system is cephalized and tetraneurous. There is a tendency to concentrate ganglia in the circumenteric nerve ring from which arise four major longitudinal nerve cords.
Molluscs may be either gonochoric or hermaphroditic. Spiral cleavage produces a veliger larva in many taxa unless it is suppressed in favor of direct development or another larva. Molluscs arose in the sea and most remain there but molluscs have also colonized freshwater and terrestrial habitats.
Eumollusca
Eumollusca, the sister taxon of Aplacophora, includes all molluscs other than aplacophorans. The eumolluscan gut has digestive ceca which are lacking in aplacophorans, the gut is coiled, and a complex radular musculature is present.
Conchifera
Conchifera, the sister taxon of Polyplacophora, includes all Recent molluscs other than aplacophorans and chitons. The conchiferan shell consists of an outer proteinaceous periostracum underlain by calcareous layers and is a single piece (although in some it may appear to be divided into two valves). The mantle margins are divided into three folds.
Ganglioneura
Most Recent molluscs are ganglioneurans, only the small taxa Aplacophora, Polyplacophora, and Monoplacophora are excluded. Neuron cell bodies are localized in ganglia.
Rhacopoda
The mantle cavity is posterior in the ancestor although it may be secondarily moved to an anterior position by torsion. This taxon includes gastropods and cephalopods.
Gastropoda C
Gastropoda is the largest molluscan taxon and is the sister group of Cephalopoda. Gastropods are united by descent from a torted ancestor although many exhibit various degrees of detorsion. Many are coiled and asymmetrical but the ancestor was probably symmetrical. Gastropods are relatively unspecialized molluscs known collectively as snails. The univalve shell, present in the ancestral gastropod and in the majority of Recent species, is reduced or lost in many representatives. The flat creeping foot was inherited from their eumolluscan ancestors but gastropods have developed a distinct head with an abundance of sophisticated sense organs. The originally posterior mantle cavity has become anterior as a consequence of torsion, although detorsion has reversed this condition in many. Gastropods were originally gonochoric and most remain so but many derived taxa are hermaphroditic. Most are marine but many taxa have invaded freshwater and the only terrestrial molluscs are gastropods. Most have a single gill, atrium, and nephridium but the most primitive representatives have two of each. Only one gonad, the right, is present. The ancestor probably had an operculum. The nervous system is streptoneurous (twisted by torsion).
Euthyneura
Euthyneurans are derived prosobranchs thought to share a common mesogastropod ancestor. Euthyneura includes the no longer recognized taxa Opisthobranchia and Pulmonata. The asymmetrical streptoneurous nervous system created by the torsion that defines Gastropoda is wholly or partly reversed by detorsion. The result is a secondarily symmetrical, euthyneurous nervous system. Euthyneurans are hermaphroditic, exhibit a tendency to reduce or loose the shell, tend to be bilaterally symmetrical, and usually lack an operculum.
Opisthobranchia sC
Opisthobranchia is a heterogeneous assortment of gastropods in several taxa. Opisthobranchia is no longer thought to be a monophyletic taxon but so far it has not been possible to decipher its evolution and relationship with other gastropods. Among opisthobranchs are found strong tendencies to detorsion, bilateral symmetry, reduction of the mantle cavity, loss of coiling, and reduction or loss of the shell. Many are sluglike. A pair of sensory rhinophores and statocysts are usually present. The group includes the pteropods, sea hares, saccoglossans, nudibranchs, and many others. All are marine.
Anaspidea sO
Sea hares are the largest opisthobranchs with some reach 60 cm in length and 2 kg in weight. The shell is lost or greatly reduced and internal. The body is more or less bilaterally symmetrical. A mantle cavity, gill, and parapodia are present. There is no crystalline style or style sac. Detorsion has resulted in a complex rearrangement of structures in the mantle and below the shell. The nervous system is detorted. The hemal system consists of heart, vessels, and large spacious hemocoelic sinuses. Sea hares are shallow water herbivores that consume seaweeds.
Laboratory Specimens
The common smooth-bodied sea hare of the United States east coast is Aplysia brasiliana (= A. willcoxi). Another species, the ragged sea hare, Bursatella leachi, also occurs on this coast but has conspicuous, soft, dendritic processes extending from the body wall that give it a shaggy appearance. Any species of sea hare can be used with this exercise.
Aplysia occurs in a variety of color patterns that correlate with the seaweed upon which it is feeding and living. The color of an individual varies with its diet. The color descriptions in the following account refer to living specimens. Preserved specimens will often be different.
Aplysia is as good an example as any of the very heterogeneous opisthobranchs. It is partially detorted and has a reduced internal shell. Like other opisthobranchs it is hermaphrodite.
Relaxation and Anesthetization
Sea hares must be relaxed and anesthetized prior to dissection. In the absence of relaxation, the muscles contract the animal into unnatural shapes that make it nearly impossible to study and understand its anatomy. Relaxation must be accomplished slowly and without disturbing the animal to avoid partial contraction.
Aplysia is difficult to relax as it tends to become misshapen in the process. Relaxation is best accomplished slowly by dripping concentrated magnesium chloride into a small container holding the animal. The concentrated solution is made by using 75 g magnesium chloride per liter of water. The solution is added slowly to the holding container using a drip string or a very slowly dripping siphon (see Laboratory Techniques chapter). The process should take several hours (overnight) and the hares should not be disturbed during that period.
External Anatomy
Examine a relaxed (or preserved) specimen of Aplysia brasiliana. Aplysia is heavy bodied, thick, and highly contractile. The body consists of head, foot, and visceral mass. It is partially detorted, is not coiled, and is superficially bilaterally symmetrical.
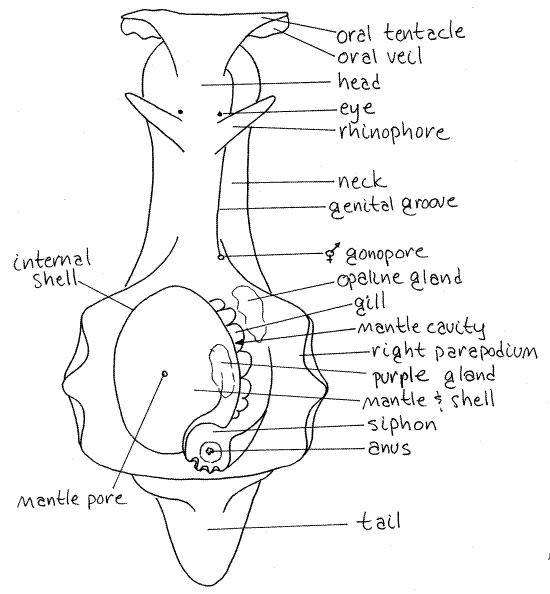
The head is located at the anterior end and is connected to the ventral foot and dorsal visceral mass by a neck (Fig 1, 12-36A). Orient the animal and be sure you know anterior, posterior, dorsal, and ventral.
Head
The head bears an anterior pair of broad, flat oral tentacles made from a fold of the body wall. The median union of the two tentacles forms a transverse oral veil over the mouth. Themouth is terminal and is located on the midline in a shallow cleft in the anterior margin of the head.
A pair of conical rhinophores project dorsally from the surface of the neck. The oral tentacles and rhinophores are sensory and, since both are covered by the same type of epithelium, probably have the same or similar function.
A small inconspicuous eye is located at the base of each rhinophore, often surrounded by a clear area but sometimes obscured by surface pigmentation. It is anteroventral to the base of the rhinophore in a tiny pit.
Figure 1. Dorsal view of a generalized Aplysia. Redrawn from Kandel, 1979. opist38La.gif
Foot
A flat, narrow creeping foot lies along the midline of the ventral surface. Its posterior extremity is modified to form a sucker that is used to attach the posterior end of the animal while the anterior end is elevated above the substratum. The foot extends posterior to the body as a short tail.
Dorsolaterally, the foot is elaborated into two large, winglike, natatory parapodia (Fig 1, 12-36A). The parapodia are widely separated anteriorly but meet each other posteriorly.
Visceral Mass
The visceral mass is the large bulge posterior to the neck, anterior to the tail, and between the parapodia. It occupies most of the central interior region of the animal and contains most of the viscera, including the digestive, excretory, hemal, and reproductive systems in the hemocoel. The internal shell covers the center of the visceral mass. Some of the viscera can be seen through the thin body wall around the shell, especially on the left.
Mantle
Between the two parapodia, on the dorsal side of the animal, is the reduced mantle and mantle cavity. The mantle is the dorsal body wall of the visceral mass. The shell is embedded in it. All that remains of the mantle is this thin layer over the shell and the nearby epithelium.
Shell
Locate the reduced, internal shell (Fig 1, 12-36A). It is covered by the mantle. In young individuals there is a large opening, the mantle aperture, in the center of the mantle over the shell. In adults, this foramen is reduced to a tiny mantle pore located on a small papilla, a little to the left of the dorsal midline. The shell is calcified centrally, but flexible peripherally.
In juveniles, the shell is clearly visible through the mantle aperture. In adults it cannot be seen from the surface but is easily palpated through the thin mantle. Its oval outline is clearly visible through the thin mantle.
Mantle Cavity
A shallow fold of the mantle overhangs and partially encloses the mantle cavity on the right (Fig 1, 12-36A). The mantle cavity is reduced and small and is confined to the space below the overhang of the shell. It opens broadly to the right and contains the gill, anus, and gonopore. Place the specimen on its left side so you can look into the mantle cavity. The partial detorsion of sea hares has moved the posterior mantle cavity of the mesogastropod ancestor to the right side.
Gill
On the right side of the shell, and mostly under its overhang, is the single large plicate (folded) gill (Fig 1, 12-36A). The gill is branched and highly folded (Fig 12-36B). The branched stem that attaches the gill to the body is hollow and contains the afferent and efferent branchial vessels bringing blood to and from, respectively, the respiratory surface. This gill, while located on the right, is homologous to the left gill of the mesogastropod ancestors of opisthobranchs.
Siphon
The mantle at the posterior right corner of the shell is folded to form a short, tubular, exhalant siphon that encloses the anus (Fig 1, 12-36A). The respiratory current enters the right mantle cavity anteriorly, between the shell and the right parapodium. It flows posteriorly over the gill surfaces to the anus and then exits posteriorly through the siphon.
Anus
The anus is in the center of the siphon at the posterior end of the mantle cavity (Fig 1, 12-36A). The nephridiopore is located here also, but is very small and probably will not be seen.
Genital Groove
The genital groove (or seminal groove, or external autospermal groove) is a long, oblique, ciliated groove (Fig 1, 12-36A) on the right dorsal side of the neck and head. It extends anteriorly from the gonopore to a penis aperture ventrolateral to the right oral tentacle. It is a part of the reproductive system to be discussed later. It is a part of both the male and the female systems of these hermaphroditic animals. It functions in sperm transfer during copulation and in egg transport during oviposition.
Penis Sheath Pore
The penis sheath pore below the base of the right oral tentacle is the opening to the penis sheath. The eversible penis is normally contained within it. The genital groove runs to the tip of the everted penis. The penis is usually retracted except when in use but in moribund, stressed, partly relaxed, or dead individuals it may be extended. In some specimens the penis may be extended from this pore. If it is extended in your specimen, trace the genital groove to its terminus. The penis in this species is long, tapered, and white.
>1a. Place a drop of carmine-seawater suspension on the genital grove and see if the ciliary transport system is operating. Does the carmine move? In which direction? Does the direction of the ciliary current give you any indication of the type of sperm, autosperm or allosperm, it transports? <
Gonopore
Trace the genital groove posteriorly into the mantle cavity to its posterior terminus. Examine this area carefully, perhaps with a dissecting microscope, and find the gonopore. This is the external opening of the hermaphroditic reproductive system. It is near the anterior end of the mantle cavity on the right side (Fig 1, 12-36A). It is surrounded by a large fleshy hood. Remember its location and avoid destroying it.
Purple Gland
The purple gland (= mantle gland) is located in the right edge of the mantle, dorsal to the right edge of the shell (Fig 1, 12-36A). Look in the mantle cavity under the shell overhang on the right side for the numerous openings of this gland. If you press gently in this area some of the purple secretion of this gland may be released from the pores, rendering them more easily seen. The purple secretion is composed of unstable chromoproteins and is not permanent. It is thought to be used to produce an opaque cloud to confuse a predator while the animal makes its escape. The location of the openings of the purple gland in the respiratory current facilitates dispersal of the ink.
Opaline Gland
The opaline gland also opens in this area. In Aplysia brasiliana this gland opens by a single duct and pore, but in many Aplysia species it has multiple openings. The opaline gland is an irregular, granular, white gland that is visible externally in the floor of the mantle cavity in the vicinity of the gonopore (Fig 1, 12-36A). Its pore is located ventral to the gonopore. During dissection, living specimens often release the secretions of the gland when the region of the pore is touched.
The viscid greenish white secretion of the opaline gland is thought to be repugnatorial but in A. brasiliana, unlike other species, it does not have an unpleasant odor. It contains toxins from the algal diet of the hare.
Figure 2. Dorsal dissection of Aplysia brasiliana from Ft. Macon, North Carolina. Opist39La.gif
Internal Anatomy
" Use fine scissors to begin the dissection of Aplysia with a mid-dorsal, longitudinal incision starting between the two rhinophores. Cut carefully through the body wall, noting its highly fibrous nature as you work. Throughout this dissection, look for muscle bundles, note their origins and insertions, and speculate about their action.
Be careful as you cut through the body wall that you do not damage the structures ventral to it. Portions of the gut lie very close to the body wall and may even adhere to it.
Extend the incision anteriorly to the mouth and posteriorly to a point beside the gonopore. Do not cut the internal duct extending posteriorly from the gonopore. Deflect the body wall, tearing muscle and connective tissue as necessary. Be careful that you do not tear the nerves which are numerous and conspicuous.
Within the body wall is a spacious hemocoel that may remind you of the coelomic cavity of vertebrates in its roominess. It is not, of course, a coelomic space, rather it is a blood sinus in the connective tissue compartment. This large open area makes this dissection an easy one.
Nervous System
The nervous system should be studied first, before it is destroyed by subsequent dissection of other systems.
The largest and most conspicuous feature in the hemocoel is the gut, which will not be studied in detail until later. At present, however, you need to be able to recognize a few regions of the gut to help you find the parts of the nervous system.
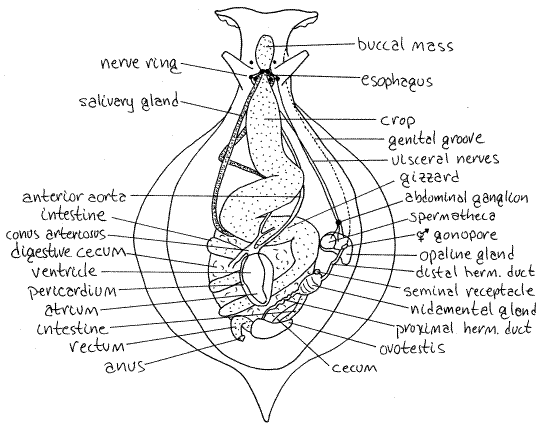
Anteriorly find the large, muscular, bulbous, yellowish-orange buccal mass (Fig 2). The mouth opens into it anteriorly and a short, narrow esophagus exits it posteriorly. The esophagus quickly expands to form the very large, sinuous, thin-walled crop.
Figure 3. Diagrammatic representation of the nerve ring of Aplysia. Opist40L.gif
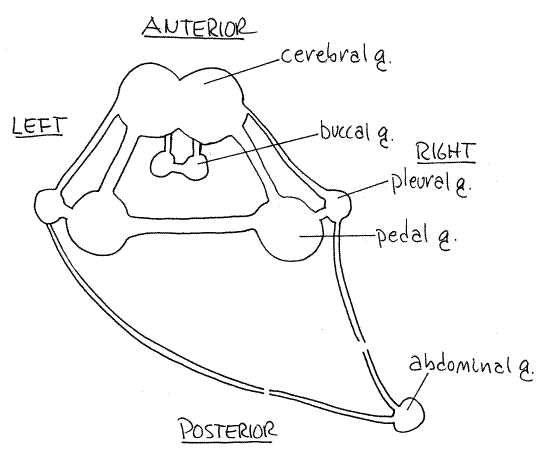
Immediately posterior to the buccal mass the two fused cerebral ganglia lie atop the esophagus (Fig 3). They, like the other ganglia in living Aplysia, are orangish-yellow due to neuroglobin in the tissues. From them extend numerous major sensory nerves from structures in the anterior part of the body. Along with other ganglia they contribute to the formation of thecircumesophageal nerve ring around the esophagus (Fig 2, 3).
On either side of the esophagus is a small pleural ganglion and a larger pedal ganglion very close and ventral to it. Each is connected to the cerebral ganglion on its side by its own connective (cerebropleural and cerebropedal connectives, respectively). Further, the pedal and pleural ganglia are connected to each other by a very short connective (pleuropedal connective). A single pedal commissure running ventral to the esophagus connects the right and left pedal ganglia with each other and completes the circumenteric nerve ring.
A pair of buccal ganglia on the buccal mass connect with the cerebral ganglia via cerebrobuccal connectives (Fig 3).
From each pleural ganglion arises a long connective that runs posteriorly to the abdominal ganglion. The two connectives are asymmetric. The composition of the abdominal ganglion is uncertain but it probably includes the visceral and esophageal ganglia. The single abdominal ganglion it is located on the right side just inside the thin body wall of this region and close to the gonopore (Fig 2). The connective from the left pleural ganglion passes under the gut, across the midline, and then runs with the right connective to the abdominal ganglion.
Trace the nerves from the cerebral, pleural, and pedal ganglia to their targets to learn which organs they innervate.
The abdominal ganglion is the center for autonomic (visceral) motor functions including respiration, excretion, reproduction, and circulation. Three major nerves arise from the abdominal ganglion to serve the respiratory structures (mantle, gill, and siphon), the viscera (nephridium, posterior gut, and heart), and the reproductive system and digestive gland.
The cerebral ganglia are primarily sensory and receive input from the rhinophores, oral tentacles, mouth, and eyes. The pleural ganglia send long connectives to the abdominal ganglion. The pedal ganglia send motor nerves to the foot, head, penis, and parapodia. The buccal ganglia are motor ganglia that control the numerous muscles of the buccal mass, the pharynx, esophagus, salivary glands, crop, and gizzard.
Digestive System
" Make a shallow, median, longitudinal, dorsal incision through the mantle on the top of the shell and gently pull the shell completely out of the mantle. Do not cut into the tissue below the shell at this time as the heart and nephridium are located here. Note the fragile nature of the shell and that there is a shallow indentation, the anal sinus, along its postero-lateral corner.
" Extend your earlier, median, dorsal body wall incision posteriorly around the left side of the visceral mass to the posterior end of the mantle cavity. Curve the incision around the posterior end of the mantle cavity and then continue it anteriorly on the right side of the visceral mass almost to the gonopore. This cut should lie between the right parapodium and the visceral mass along the dorsal edge of the parapodium. Be careful that you cut only the body wall and not the organs, ducts, or nerves lying below it. Deflect the dorsal body wall (mantle) to reveal the hemocoel below it.
Free the organs of the hemocoel from connective tissue and muscle as necessary to expose the gut beginning at the anterior end of the body.
Most of the gut is delicate and must be handled carefully to avoid tearing its walls. Anteriorly there is the large, orangish or yellowish buccal mass which contains the buccal cavity, jaws, pharynx, radula and odontophore (Fig 2). You will open it soon, but at present you can see the vague dark outline of the pharynx tube through the thin muscle on top of the buccal mass. The color of the buccal mass is imparted by myoglobin contained in its muscles.
From the posterior end of the buccal mass extends the short, narrow esophagus. Flanking the base of the esophagus is a pair of very long, narrow salivary glands (or pharyngeal glands, Fig 2). These arise from the pharynx in the posterior part of the buccal mass and extend far back in the visceral mass where they entwine with the crop. The epithelium of the salivary glands consists solely of mucus- producing cells. The locations of the two salivary glands are asymmetrical.
" Before proceeding with the remainder of the gut, open the buccal mass with a shallow, mid dorsal, longitudinal incision running from mouth to esophagus. Avoid damaging the nerve ring.
The lumen of the buccal mass is the buccal cavity (in the anterior part of the buccal mass) and the pharynx (in the posterior part). The two regions are separated by a delicate veil of tissue.
Look at the mouth, which you probably did not see clearly prior to dissection. It is encircled by a lip. The mouth opens into the buccal cavity which contains the dark jaws composed of parallel, cuticular rods.
The larger pharynx lies posterior to the buccal cavity. Below the pharynx is the large odontophore with its many specialized muscles. The radula lies atop the odontophore and is supported by it. In this species the radula consists of about 65 - 70 rows of tiny teeth. The salivary glands arise from the posterior pharyngeal cavity.
>1b. Make a wholemount of a small piece of the radula and examine it with the compound microscope. <
The short esophagus leads from the pharynx to a very large and very thin-walled crop (Fig 2). The crop is dull gray-brown, but its contents can be seen through the thin walls and may affect the apparent color. It is a storage area and will be filled with algae if your animal has been feeding recently. The crop curves first to the right, back to the left, and then loops under itself and heads toward the right again. The salivary glands extend into the space between the turns of the crop.
Before the crop reaches the midline on its last twist to the right it becomes the more muscular, thicker walled, and triturative gizzard (Fig 2). This is oriented transversely or obliquely across the midline.
" Open the gizzard with a longitudinal incision and examine the numerous large, non-calcareous, conical teeth on its inner walls. The teeth are secreted by rhomboidal patches of glandular epithelium. If the teeth are dislodged or absent, the epithelial patches will be seen instead. The teeth are easily dislodged.
Posterior to the gizzard and on the right side is the short stomach. This region is also fairly thin-walled but not as thin as the crop.
The stomach extends posteriorly but soon constricts to form the smooth, gray-brown, thin-walled intestine. The intestine is embedded in the large gray-brown digestive cecum. The intestine makes several coils through the digestive cecum and the last coil is located on the right posterior edge of the digestive cecum (and visceral mass). This coil turns toward the midline, from the right, and then turns posteriorly to terminate at the anus, located in the siphon between the mantle and body wall below the posterior overhang of the shell. The posterior intestine is overlain by the pale brown or orangish ovotestis.
A long diverticulum, the gastric cecum, arises from the stomach and extends posteriorly embedded in the digestive cecum. It is pinkish but mostly is visible only at its posterior end near the orange ovotestis in the posterior visceral mass (Fig 2). You may not see it. The gastric cecum has a ciliated typhlosole within it.
Reproductive System
Anatomy
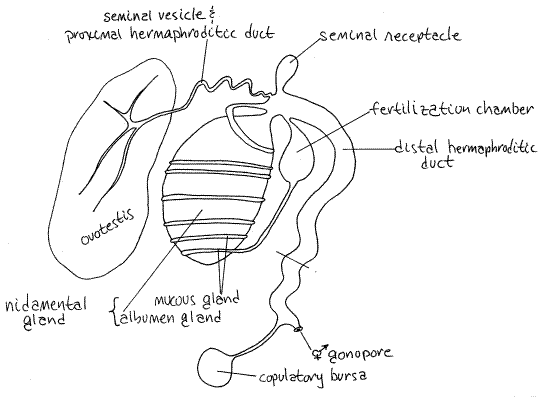
Opisthobranchs are simultaneous hermaphrodites with a combined ovotestis and single hermaphroditic gonoduct. In Aplysia the gonoduct has male and female channels, separated by a typhlosole, within it (Fig 4, inset). Evolutionarily and developmentally the internal system is derived from the coelom and the mantle.
Figure 4. Diagrammatic representation of the reproductive system of Aplysia. Based on several sources and living specimens. Opist41La.gif
You have already seen the genital groove, gonopore, and penis sheath pore. Look now inside the head to the right of the buccal mass to find the pale, yellowish, muscular penis sheath. It contains the retracted penis and connects with the penis sheath pore but neither it not the penis are connected with the gonoduct. Sperm are delivered to the penis by the genital groove.
Relocate the gonopore. The internal hermaphroditic gonoduct opens to the exterior here. "
Most the reproductive system is pale and is easily located. Cut the thin body wall as necessary to trace the gonoduct posteriorly from the gonopore. Eventually you will reach the orange or brown ovotestis at the posterior edge of the digestive cecum at about the level of the anus (Fig 2). Its size varies with the maturity and reproductive condition of the individual.
The duct from gonopore to ovotestis is the hermaphroditic duct and it is divided into proximal and distal portions (Fig 4, 12-59B). Both transport eggs and sperm.
The proximal hermaphroditic duct (or small hermaphroditic duct) leaves the ovotestis. It is narrow in immature individuals but in mature specimens is wider and convoluted to form aseminal vesicle for storage of autosperm. In mature specimens it is creamy-white due to the presence of sperm. The seminal vesicle is the proximal hermaphroditic duct itself and is not a diverticulum. Autosperm are inactive until ejaculated into a partner.
The proximal hermaphroditic duct enlarges in diameter to become the distal hermaphroditic duct (Fig 4). Near this junction are locate the nidamental gland and seminal receptacle. The complex nidamental gland is a large, ovoid, greenish, cream, yellow, or orangish organ containing the winding gland, mucus gland, albumen gland and fertilization chamber (Fig 4). You will not be able to distinguish all these parts.
A small gray or white seminal receptacle for storage of allosperm is attached to the duct near the point where the proximal hermaphroditic duct joins it. Allosperm area active, with undulating flagella.
Figure 5 Cross section of the distal hermaphroditic duct of Aplysia showing the three channels.
The distal hermaphroditic duct (= large hermaphroditic duct) extends from the nidamental gland to the gonopore. It is shorter and wider than the proximal duct and in cross section can be seen to contain three channels (Fig 5). It transports eggs and both types of sperm, allosperm and autosperm. It also functions as a vagina for reception of the partner's penis. Just before it reaches the gonopore it receives the relatively long duct from the translucent or brown, spherical copulatory bursa (or gametolytic gland, or spermatheca). This gland probably destroys superfluous sperm.
Mating
Sea hares are simultaneous hermaphrodites that cross-fertilize by copulation. When two, or sometimes several, individuals copulate, each participant donates and receives sperm to and from another individual, but not necessarily the same individual.
During copulation the penis of one individual is inserted into the gonopore of its partner and the gonopore of the former receives the penis of the latter or of yet a third individual (Fig 12-59A). The penis is erected by engorgement with blood. It extends into the vaginal channel (Fig 5) of the distal hermaphroditic duct of the recipient to the opening of the seminal receptacle.
The donor’s penis is inserted in the gonopore of the recipient and into the vaginal channel of the distal hermaphroditic duct. Sperm are ejaculated from the donor's seminal vesicle by peristaltic muscular contractions. The sperm move downstream through the autosperm channel (Fig 5) of the distal hermaphroditic duct by ciliary currents. They exit the gonopore and travel along the genital groove to the tip of the erect (donor) penis.
The sperm exit the penis and enter the duct of the recipient's seminal receptacle where they are stored. These sperm are active and capable of fertilization. The walls of the receptacle are muscular. The reciprocal release of sperm occurs in the opposite direction. The two (or more) partners separate each now with a supply of allosperm stored in its seminal receptacle.
Oviposition
Later, during oviposition, large numbers of oogonia leave the ovotestis and move through the proximal hermaphroditic duct (Fig 4, 12-59B). Here they begin, but do not complete their first meiotic division and become oocytes. Oocytes pass the opening of the albumen gland from which they receive a coating of nutritive albumen (Fig 4). At this time allosperm are ejected from the seminal receptacle to mix with the oocytes and the mixture enters the fertilization chamber (Fig 4). Fertilization of the oocytes begins here.
The mixture moves along the duct of the winding and mucus glands where thick layers of mucus and jelly are applied. Numerous (about 60) oocytes are formed into a jelly-covered string which enters the oviducal channel of the distal hermaphroditic duct (Fig 5) and receives the adhesive secretions of its epithelium. The strings exit the gonopore and travel along the genital groove to be deposited on the substratum.
The emerging strings form a spaghetti-like mass on the substratum (Fig 12-59C,D). Fertilization of the oocytes is still occurring. Follwoing fertilization the oocytes complete their meiotic divisions within a few hours of oviposition and become ova. The male and female pronuclei combine to form a zygote nucleus and typical spiralian cleavage ensues. A planktotrophic veliger eventually hatches from the egg string.
> 1c. If Aplysia have been maintained in the laboratory for a period of time there may be egg strings in the holding tank. Remove some to a smaller container. Examine a few to determine the stage of the embryos. Keep some eggs in a small container until veligers emerge or examine the water of the holding tank for veligers. <
Hemal System
Anatomy
" Relocate the as yet undisturbed portion of the visceral mass below the shell. Remove the shell if you have not already done so and cut through the roof of the visceral mass to expose the tissues below. The space revealed by this incision is the coelom. It is subdivided into a pericardial cavity on the anterior left side and a nephridium, or kidney, on the right.
The two subdivisions of the coelom are connected by a broad, open, short renopericardial canal. The pericardial cavity is enclosed by a membranous pericardium and contains theheart which consists of a large, thick-walled ventricle and a short, thin-walled atrium (Fig 2). The efferent blood vessel from the gill enters the atrium.
The ventricle empties into a large conus arteriosus from which three aortae diverge and leave the pericardial cavity (Fig 2). The left and largest of the aortae enters the digestive cecum between two loops of the intestine. Trace it if you wish. The middle aorta runs anteriorly to the gizzard and crop. The right aorta passes by the copulatory bursa and then runs anteriorly through the hemocoel to the buccal mass. It is a long, straight, transparent vessel.
> 1d. Inject concentrated dye into the conus arteriosus to help visualize these aortae and their branches. Note the large closed sac at the junction of the ventricle and aorta. This sac may be involved in production of the ultrafiltrate. <
Circulatory Pattern
The hemal system is open, with arteries from the heart opening into spacious hemocoelic sinuses. From these spaces venous blood enters vein-like channels eventually leading back to the heart. Blood collects in the main abdominal hemocoel and then passes either to the gill or the kidney.
Some blood goes to the gill in an afferent branchial vessel that ramifies through the folds and branches of the gill. It is oxygenated and then leaves the gill by an efferent branchial vessel that crosses the pericardium and empties into the atrium of the heart.
Blood that went to the kidney, instead of the gill, flows over the nephridium where its composition is modified by absorption and secretion from and to the nephridium. It then joins the efferent branchial flow from the gill and enters the atrium.
The heart thus receives some blood that is oxygenated and some that is modified by the kidney. The atrium empties into a muscular ventricle that forces blood anteriorly into an aorta that branches into arteries to various parts of the body.
Excretory System
The single nephridium is located on the right between the pericardial cavity and the gill. It is a very large, thin, pink structure below the left side of the shell. It is a very large, thin, pink structure below the left side of the shell. The renopericardial canal connects the nephridium with the pericardial cavity and is ciliated. The nephridium empties via a duct to the nephridiopore beside the anus.
The pressure imparted on the blood by the ventricle forces primary urine (ultrafiltrate) across the walls of the heart and into the pericardial cavity. Here it is modified by selective absorption and secretion vis a vis the blood outside the nephridium and becomes the final urine. The urine is expelled through the nephridiopore.
Behavior
Opisthobranch molluscs creep rapidly using the foot which is fitted with longitudinal and dorsoventral smooth muscles. Transverse waves of contraction move along the sole of the foot. In Aplysia, but not in other opisthobranchs, the waves are prograde; moving from posterior to anterior, in the direction of motion. The creeping speed of Aplysia is greater than that of other opisthobranchs and is about 50 cm/min (Thompson, 1976).
Observe Aplysia crawling and measure its speed.
Aplysia is capable of swimming using the two parapodia (Fig 12-37). Synchronized waves of muscular contraction pass from anterior to posterior to produce a graceful swimming motion with almost no roll (Thompson, 1976). This characteristic motion is reminiscent of the swirling skirts of flamenco dancers and has resulted in the colloquial name "Spanish dancer" in some parts of their range. When the animal is in creeping mode, the parapodia are often folded over the body and their undulations ventilate the mantle cavity and gill. Swimming by Aplysia is facilitated by the extreme reduction of the shell and resulting decrease in weight.
> 1e. Observe a healthy living Aplysia in a tank of seawater. Note the manner in which it uses its foot to creep along the substratum. Measure its speed.
Watch the animal swim using the parapodia on the sides of the body. Does the motion appear to be well coordinated or haphazard? Are the right and left parapodia in synchrony with each other? Do they move in unison with each other or alternately? <
References
Eales NB. 1960. Revision of the world species of Aplysia (Gastropoda, Opisthobranchia). Bull. British Mus. (N.H.), Zool. 5(10):269-404.
Howells HH. 1942. The structure and function of the alimentary canal of Aplysia punctata. Quart. J. Micros. Soc. 83:357-397.
Hyman LH. 1967. The Invertebrates. vol. VI. Mollusca. McGraw-Hill, New York, 792 pp.
Kandel ER. 1979. Behavioral biology of Aplysia. W.H. Freeman, San Francisco. 463 pp.
Ruppert EE, Fox RS, Barnes RB. 2004. Invertebrate Zoology, A functional evolutionary approach, 7 th ed. Brooks Cole Thomson, Belmont CA. 963 pp.
Thompson TE. 1976. Biology of Opisthobranch Molluscs, vol 1. Ray Society, London, 206 pp.
Supplies
Aplysia, living or preserved
Concentrated magnesium chloride
Dissecting pan (about 20 X 30 cm)
Dissecting set
Carmine seawater suspension