Invertebrate Anatomy OnLine
Actinonaias ©
Freshwater Unionoid Mussels
25may2007
Copyright 2005 by
Richard Fox
Lander University
Preface
This is an exercise from Invertebrate Anatomy OnLine , an Internet laboratory manual for courses in Invertebrate Zoology. Additional exercises, a glossary, and chapters on supplies and laboratory techniques are also available. Terminology and phylogeny used in these exercises correspond to usage in the Invertebrate Zoology textbook by Ruppert, Fox, and Barnes (2004). Hyphenated figure callouts refer to figures in the textbook. Callouts that are not hyphenated refer to figures embedded in the exercise. The glossary includes terms from this textbook as well as the laboratory exercises.
Systematics
Mollusca P, Eumollusca, Conchifera, Ganglioneura, Ancyropoda, Bivalvia C, Metabranchia sC, Eulamellibranchia SO, Unionoida O, Unionoidea SF, Unionidae F, Ambleminae sF (Fig 12-125, 12-122)
Mollusca P
Mollusca, the second largest metazoan taxon, consists of Aplacophora, Polyplacophora, Monoplacophora, Gastropoda, Cephalopoda, Bivalvia, and Scaphopoda. The typical mollusc has a calcareous shell, muscular foot, head with mouth and sense organs, and a visceral mass containing most of the gut, the heart, gonads, and kidney. Dorsally the body wall is the mantle and a fold of this body wall forms and encloses that all important molluscan chamber, the mantle cavity. The mantle cavity is filled with water or air and in it are located the gill(s), anus, nephridiopore(s) and gonopore(s). The coelom is reduced to small spaces including the pericardial cavity containing the heart and the gonocoel containing the gonad.
The well-developed hemal system consists of the heart and vessels leading to a spacious hemocoel in which most of the viscera are located. The kidneys are large metanephridia. The central nervous system is cephalized and tetraneurous. There is a tendency to concentrate ganglia in the circumenteric nerve ring from which arise four major longitudinal nerve cords.
Molluscs may be either gonochoric or hermaphroditic. Spiral cleavage produces a veliger larva in many taxa unless it is suppressed in favor of direct development or another larva. Molluscs arose in the sea and most remain there but molluscs have also colonized freshwater and terrestrial habitats.
Eumollusca
Eumollusca, the sister taxon of Aplacophora, includes all molluscs other than aplacophorans. The eumolluscan gut has digestive ceca which are lacking in aplacophorans, the gut is coiled, and a complex radular musculature is present.
Conchifera
Conchifera, the sister taxon of Polyplacophora, includes all Recent molluscs other than aplacophorans and chitons. The conchiferan shell consists of an outer proteinaceous periostracum underlain by calcareous layers and is a single piece (although in some it may appear to be divided into two valves). The mantle margins are divided into three folds.
Ganglioneura
Most Recent molluscs are ganglioneurans, only the small taxa Aplacophora, Polyplacophora, and Monoplacophora are excluded. Neuron cell bodies are localized in ganglia.
Ancyropoda
The mantle cavity, with its gills, is lateral. The calcareous portion of the shell is bivalve, with the valves opening laterally and joined dorsally by a derivative of the periostracum.
Bivalvia C
Bivalvia is a large, successful, and derived taxon. The body is laterally compressed and enclosed in a bivalve shell. The two valves are hinged dorsally. The the foot is large and adapted for digging in the ancestral condition. A crystalline style is usually present but never is there a radula. The mantle cavity is lateral and in most bivalves the gills are large and function in respiration and filter-feeding. The head is reduced and bears no special sense organs. The nervous system is not cephalized. The group includes scallops, clams, shipworms, coquinas, marine and freshwater mussels, oysters, cockles, zebra mussels, and many, many more.
Metabranchia sC
Metabranch gills are adapted for filter feeding. Water enters the mantle cavity posteriorly.
Eulamellibranchia SO
Eulamellibranchs have gills with tissue interfilamentar connections.
Unionoida O
(Paleoheterodonta)
The lamellar layer of the shell is nacreous (pearly). The apertures are poorly developed and the shells are equivalve. Modern classifications of unionoid mussels recognize two families in North America; Margaritiferidae and Unionidae. Two subfamilies of unionids, Ambleminae and Unioninae, are recognized. Most amblemines are North American where they occur exclusively (or almost so) in the Atlantic and Gulf of Mexico drainages. Approximately 75% of North American genera and 80% of species belong to Ambleminae. The North American Ambleminae belong to the tribes Amblemini, Pleurobemini, and Lampsilini.
Natural History
Native North American freshwater bivalves are either mussels in the order Unionida O (Margaritiferidae F and Unionidae F) or fingernail clams (Veneroida O: (Corbiculoidea SF: Sphaeriidae F). Introduced bivalves include the now widely dispersed Asian clam, Corbicula fluminea, and the rapidly dispersing zebra mussel, Dreissena polymorpha.
Freshwater mussels (Unionoidea SF) occur worldwide but they are most abundant in North America, with their greatest diversity in the Ohio River Drainage of the Southeastern United States. They should not be confused with the unrelated marine mussels (Filibranchia SO, Pteriomorpha O) from which they differ in many important respects.
Freshwater mussels are usually found in medium to large rivers in water less than two meters deep. A few species inhabit the quiet water of lakes but most are riverine. Small creeks and small lakes have few mussels although they may have diverse faunas of other bivalves such as the sphaeriid clams. Mussels live in soft, sedimentary bottoms with the foot and anterior end of the shell and body dug into the sediment with the posterior end exposed. Adult mussels move slowly and never very far from the site of settling after leaving the fish host. Dispersion occurs as larvae parasitic on fishes. Like other bivalves, mussels are suspension feeders using the gills as filters to remove particulate organic matter, detritus and plankton, from the water. Adults unionoids can be 4-30 cm in length.
Most freshwater mussels are gonochoric and cross fertilize without copulation. Fertilization is external and occurs in the water tubes of the female demibranchs where embryos are brooded to the larval stage. This is sometimes referred to as internal fertilization but, since the water tubes are part of the mantle cavity, and not the gonoduct, it is topologically outside the body and it is probably preferable to consider it to be external. The larva is the unique glochidium, an external parasite of fishes. Glochidia live only attached to the fish host and without the appropriate fish the life cycle cannot be completed.
Our freshwater mussels are imperiled by several human activities including habitat destruction (especially the impoundment of rivers), siltation from agricultural and construction runoff, organic and chemical pollution, competition with introduced exotics such as Asian clams and zebra mussels, extirpation of fish hosts for the larvae, and direct exploitation, originally for the button industry and now as a source of beadstock for the cultured pearl industry. Of about 300 unionoid taxa once known from North America 70% are Endangered, Threatened, or of Special Concern. Twenty one taxa are Endangered and possibly Extinct, 77 are Endangered and extant, 43 are Threatened, 72 are of Special Concern, 14 are Undetermined, and only 70 taxa, or 24%, are Currently Stable. Harvesting of mussels is not federally regulated except for species on the federal list of endangered or threatened species although many states regulate species and sizes that can be harvested.
Unionoid genera differ from each other in hinge morphology, hinge tooth morphology and development, shell thickness, the shell inflation, shell ornamentation, elaborations of the mantle margin, and use of the demibranchs for brooding. Species and genera are difficult to identify.
Laboratory Specimens
Many native North American freshwater mussels are threatened or endangered and are protected by state and federal legislation. Obviously, these species should never be used for dissection in teaching laboratories. Whenever possible alternatives to native unionoid mussels should be used for the study of bivalve anatomy. Clams (Mercenaria mercenaria), marine mussels (Mytilus edulis), and oysters (Crassostrea virginica) are often available alive and inexpensively from local fish markets and supermarkets, even in the interior of the continent. If locally collected living specimens of freshwater bivalves are desired, it is much better to use the abundant, widespread, invasive Asian clam, Corbicula fluminea. Dissection instructions for each of these species are available at Invertebrate Anatomy OnLine .
To avoid further damage to endangered and threatened populations native mussels should not be collected locally for laboratory use unless the instructor can identify the specimens to species. Federal legislations provide for substantial penalties for collection or possession of Threatened or Endangered species and some states require permits. Biological supply companies provide specimens of what we assume are species without conservation concern, classified as Currently Stable, for laboratory use. The actual identity of commercially supplied specimens varies and can only be known by identifying the specimens upon arrival at your institution. The catalogs advertise them as Unio or Anodonta but these names are used sensu lato, in the broad sense. Both genera, once large, have been split into several smaller genera and as the genera are currently defined, there are no species of Unio in the Americas.
Anodonta has thin shells without hinge teeth. Other genera, including genera such as Actinonaias and Lampsilis have thick shells and hinge teeth. Both types are marketed under the designation “ Anodonta or Unio”. This exercise was prepared using preserved, commercially supplied Actinonaias (marketed under the name “ Anodonta-Unio”) but can be used with fresh or preserved specimens of any unionoid mussel. The dissection is best conducted with the specimen covered with tap water although this may prove impracticable until both valves have been removed. A dissecting microscope should be used as needed.
Although the exercise is written to be used with preserved specimens, it can also be used with living or freshly killed animals. Living specimens are very difficult to open due to the ability of the adductor muscles to hold the valves tightly together indefinitely. Literature accounts suggest placing living mussels in warm tap water (55-60° C) for a few minutes. This is said to result in relaxation of the muscles and consequent gaping of the valves. Alternatively, mussels can be boiled or steamed briefly, until the valves gape open. Any procedure that kills the specimen is, of course, incompatible with later observation of ciliary activity on the gills, labial palps, and stomach walls or beating of the heart. Once the valves have separated, a sharp scalpel can be inserted between the valves to sever the anterior and posterior adductor muscles. Living specimens should be anesthetized in 5% non-denatured ethanol in water.
External Anatomy
Shell
External Shell Features
Examine a cleaned dry shell from which the animal has been removed or, if such a shell is not available, an intact specimen with the animal still within the shell (Fig 1). The bivalve shellconsists of two halves, or valves. The plane of symmetry passes between the two valves and divides the animal into right and left sides, which in most cases are more or less mirror images of each other.
The two valves articulate with each other along the dorsal midline via a hinge mechanism which may be simple and toothless (Anodonta, Pyganodon) or complex and toothed (most unionoids). The valves are held together along the hinge by a dark brown proteinaceous hinge ligament (Fig 1, 12-92B).
The valves can move laterally, away from each other, along the anterior, ventral, and posterior margins but are always in contact along the dorsal hinge. This lateral motion opens a space, known as the gape, along the anterior, ventral, and posterior margins and thus providing the animal with access to its environment. If you have an intact preserved specimen the gape will be present and obvious, although it will probably be larger than it would be in life. If you have a living specimen the valves will be held tightly together and there will be no gape.
Figure 1. External view of the right valve of Actinonaias. Mussel146L.gif
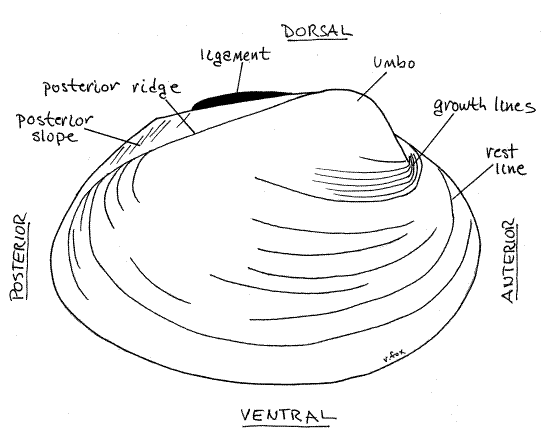
On each side of the hinge, anterior to the ligament, each valve displays a raised area known as theumbo, or beak (plural = umbones) (Fig 1, Fig 12-92B). The umbo is the oldest part of the shell. The two umbos almost touch across the midline. The umbo is anterior to the externally visible portion of the hinge ligament.
The anterior position of the umbo and its location with respect to the ligament can be used to recognize the anterior end of the clam without resorting to soft anatomy. Use the umbo to identify the anterior end of your mussel and the hinge to identify dorsal. Use these landmarks to find ventral and posterior and to determine which valve is theright valve and which is the left valve.
Relocate the ligament and note that it is outside the hinge and posterior to the umbo. In all freshwater bivalves the ligament is external. The posterior portion is obviously external and is clearly visible on the outside of the hinge even when the valves are closed. The anterior portion, although still external, is hidden when the valves are closed. The critical criterion being the position of the ligament with respect to the hinge teeth. If the ligament is outside the line of hinge teeth, it is external, if inside the line of teeth, it is considered to be internal. The ligament, being elastic, antagonizes the two adductor muscles and opens (abducts) the valves when the adductors are relaxed (Fig 12-92A). An external ligament is stretched when the valves are closed and pulls the valves apart when the adductors are relaxed. An internal ligament, on the other hand, is compressed when the valves are closed and pushes the valves apart when the adductors relax.
The two valves of a unionoid mussel are similar to each other, a condition known as equivalve. Some clams, such as the marine oysters and jingles, have very different right and left valves and are inequivalve. Furthermore, the anterior and posterior ends of freshwater mussels are more or less similar to each other and the valves are said to be equilateral. Equilateral valves are symmetrical on a dorsal ventral axis. In contrast, the two ends of some bivalves, such as the marine mussels (Fig 12-110B,E) and pen clams (Fig 12-110C) are very different, are not symmetrical, and are inequilateral although they are equivalve.
Numerous closely spaced, fine, concentric growth lines are visible on the outside surface of the valves. Larger, more widely spaced, concentric ridges are rest lines. An obliqueposterior ridge extends from the umbo in a posteroventral direction to the posterior margin of each valve. In Actinonaias the ridge is inconspicuous but in some genera it is strong and well developed. The region of the valve posterior to the ridge is the posterior slope. Depending on species, a variety of spines, corrugations, rays, or pustules may ornament the valves externally but none of these is present in Actinonaias.
If you are using cleaned valves to study, go directly to “Internal Shell Features”. If you have an intact specimen, skip to “Preview of Soft Anatomy”.
Internal Shell Features
If you have a cleaned shell, its internal features can be studied now. If you have an intact specimen, you must wait until the shell has been opened and the animal removed before you can study the inside of the valves (Fig 2, 12-92B).
Relocate the hinge region along the dorsal margin of the valve. Find anterior and posterior, dorsal and ventral.
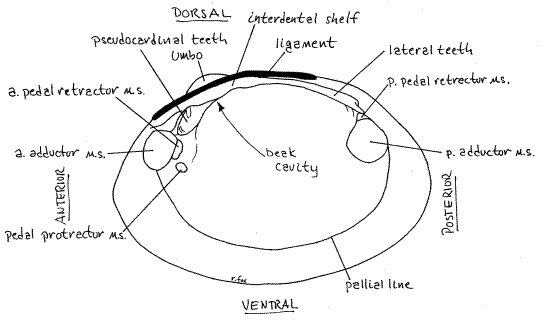
The inside surface of each valve bears scars of the muscles that attached to it. Among these are the conspicuous anterior and posterior adductor muscle scars (Fig 2, 12-92B). The anterior scar is the smaller of the two. The pallial line, along which the pallial muscles of the mantle insert, is a conspicuous line or groove paralleling the margin of the valve from the anterior adductor muscle scar to the posterior adductor muscle scar. The mantle is attached to the valve along this line. The small pedal protractor muscle scar is located near the posterior ventral margin of the anterior adductor scar. Dorsal to the protractor scar are the anterior pedal retractor muscle scars. The posterior pedal retractor muscle scar is dorsal to the posterior adductor scar and may be continuous with it or slightly separated from it. These muscles originate on the shell and insert on the foot. Their action is to help extend the foot out of the gape (pedal protractors) or withdraw the foot into the mantle cavity (pedal retractors).
The umbo rises above the hinge and the ligament extends posteriorly from the umbo. Most unionoid hinges are equipped with hinge teeth to prevent shearing and keep the valves aligned when the powerful adductor muscles pull them together. The teeth are the pseudocardinal teeth and the lateral teeth. The teeth of the right and left valves are not symmetrical. Instead a tooth on one valve opposes, and fits snugly into, a depression on the opposite valve.
The jagged, massive pseudocardinal teeth are far anterior, near the anterior adductor muscle scar. Actinonaias, like most unionoids has one large pseudocardinal tooth in the right hinge and two in the left.
The lateral teeth are on the posterior end of the hinge and are long smooth ridges. The right hinge has one lateral teeth and the left hinge has two. The pesudocardinal teeth look a little like vertebrate teeth but the laterals do not. Compare the left and right valves to demonstrate to yourself the way the teeth fit together and prevent shearing.
Figure 2. Internal view of a right valve of Actinonaias. a. = anterior, p. = posterior, m.s. = muscle scar. Mussel147L.gif
Anterior lateral teeth are absent in mussels and some lack teeth altogether (viz. Anodontinae; Anodonta, Pyganodon). The Asian clam, Corbicula, and the sphaeriid clams (both CorbiculoideaSF) have true cardinal teeth preceded and followed by anterior and posterior lateral teeth, respectively. In unionoids true cardinal teeth are absent and the ancestral anterior laterals have been modified to form the pseudocardinals.
Mollusc shells are secreted in layers by the mantle epithelium. The three layers of the typical bivalve shell are present and well developed in mussels. All three layers are secreted by the mantle, each by a specific region of secretory epithelium.
The outermost layer is the proteinaceous periostracum. In unionoid mussels its color varies but is usually black, brown, or yellow (Fig 12-91). It may be patterned, often with rays. In older individuals the periostracum is often damaged or eroded and much of it may be absent, especially near the umbo.
The innermost layer of the shell, the one adjacent to the animal itself, is the lamellar layer (= hypostracum, Fig 12-91) composed of thin calcareous sheets, or lamellae, of nacre(pronounced NAKE ur), or mother of pearl, layered on top of each other. The shiny lustrous nacre is visible on the inside of the valve. Most of the inside surface of the valve, except for the extreme outer border, is covered by nacre. The color of the nacre varies with species.
The calcareous, white prismatic layer (= ostracum, Fig 12-91) lies between the periostracum and lamellar layers. It can be seen externally in areas near the umbo where the periostracum is eroded, exposing the white prismatic layer beneath it. It is composed of vertical prisms, polygonal in cross section.
The periostracum is impermeable to water and its presence protects the underlying calcium carbonate layers from dissolving and being eroded by the surrounding water. Its presence at the edge of the shell is essential for the continued secretion of new prismatic layer. It seals the margin and creates a chemically regulated extrapallial space in which new shell can be precipitated (Fig 12-91). In older areas of the shell, i.e. nearer the umbo, the protective periostracum is often broached and the prismatic layer may be exposed and subsequently eroded.
> a. With the dissecting microscope examine a broken shell of a mussel, or other large bivalve such as Mercenaria, and view the shell layers in cross section. The piece you examine should include the original edge of the shell. The periostracum forms a thin organic layer on the outer surface. The prismatic and lamellar layers are distinctly different in this view. Theprismatic layer, which lies immediately below the periostracum, is composed of conspicuous, more or less vertical, closely spaced columns, or prisms, of calcium carbonate. As you know, the prisms are vertical to the surface of the shell. The lamellar layer on the other hand consists of more or less horizontal sheets lying inside the prismatic layer. These lamellae are parallel to the surface of the shell. Note that the lamellar layer extends almost to the edge of the shell but that closest to the edge only the prismatic layer is present. The extreme edge is peripheral to the area where the lamellar layer is being secreted and consists entirely of recently secreted periostracum and prismatic layer with no lamellar layer yet present (Fig 12-91). <
Preview of Soft Anatomy
Commercially supplied preserved specimens will arrive gaping, usually with a wooden peg holding the valves apart in a wide ventral gape. The major features of the external soft anatomy can be previewed through this gape before a valve is removed. With living specimens this preview must wait until the adductor muscles have been cut. Look into the gape and note the most obvious features for use later as landmarks. You may have to force the valves a little farther apart to improve your view but, if so, be careful that you do not tear or otherwise damage the soft tissues.
The mantle, a molluscan apomorphy, is the dorsal body wall which typically exhibits modification in different ways in different classes. Under each of the two valves of a bivalve the mantle forms a thin sheet of soft tissue. These two sheets are the right and left mantle skirts (also known as mantle lobes or mantle folds by other authors) and they enclose the animal like a cloak (i.e. mantle) (Fig 12-90). The two skirts are joined to each other and to the body along the dorsal midline, under the hinge. Laterally and ventrally the space enclosed by the two skirts is the mantle cavity. In life it is filled with circulating water. The circulation is driven by cilia on the gills. Posteriorly the margins of the right and left mantle skirts conspire to form a pair of openings, the ventralinhalant aperture and the dorsal exhalant aperture. Species of Unionidae and Margaritiferidae have simple apertures rather than siphons. Among the Unionoida only Mycetopodidae, Iridinidae, and Hyriidae have siphons.
In the center of the mantle cavity is the laterally compressed, anteriorly directed foot, which in preserved or dissected living specimens will be strongly contracted with a wavy ventral edge. The large mass of tissue dorsal to the foot is the visceral mass in which are located most of the organ systems including gonad, kidney, heart, and gut.
The bivalve head is weakly developed and lacks the concentration of sensory equipment typical of the heads of other molluscs. In bivalves most sensory receptors are located in the mantle margin, especially in the vicinity of the apertures.
Figure 3. View of the external soft anatomy of the right side of an undissected specimen. The right valve and mantle skirt have been removed resulting in the partial opening of the right exhalant chamber and exposure of a few water tubes belonging to the lateral demibranch. Because this is a preserved specimen tissues are unnaturally contracted. a = anterior, m = muscle, p = posterior. Mussel149L.gif
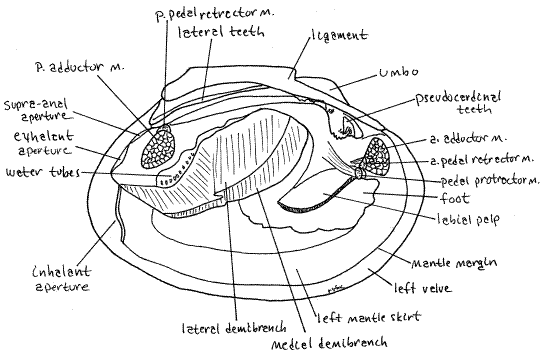
On each side of the visceral mass, between the mass and a mantle skirt, is a long, leaf-like gill. It may seem to you that there are two gills on each side but there is actually only one. At the anterior end of each gill there is a smaller, but also leaf-like labial palp. The palps resemble miniature gills and like them are double but there is actually only one on each side. Immediately anterior to the foot and labial palps is the anterior adductor muscle extending transversely from valve to valve. Hold the specimen so you can look into the posterior end of the mantle cavity. Here you will see the posterior adductor muscle extending across the cavity from valve to valve. The action of the two adductor muscles is to bring the two valves together (adduct the valves).
Soft External Anatomy
" After previewing the soft anatomy remove the right valve as follows. Identify the right valve and then look into the gape and find the anterior and posterior adductor muscles. With a scalpel or blunt probe scrape the two adductor muscles away from their attachment to the right valve. This will be easy to do in preserved specimens, in fact, the adductors may already be separated from their attachments. This process should also separate the protractor and retractor muscles from the valve. (The adductors of fresh specimens must be cut with a sharp scalpel. The posterior muscle should be cut immediately to the right of the rectum. Be careful that you do not cut any tissues other than the two muscles.)
Separate the margin of the right mantle skirt from the periostracum that attaches it to the right valve. Gently lift the right valve away from the left, leaving the right mantle skirt behind, with the remainder of the mussel. Use the blunt, flat handle of the scalpel as necessary to push, not cut, the mantle skirt away from the inner surface of the right valve. You will notice that the right mantle skirt is attached to the right valve, not only by the periostracum along its edge, but also by a line of pallial (pallial = mantle) muscles that parallels the valve margin. As you break the connection of the pallial muscles with the valve note that a distinct pallial line (Fig 2, !2-92B) marking the former attachment site is thereby revealed. All soft tissues, including the right mantle skirt, should remain cradled in the left valve. Break or cut the ligament and remove the right valve.
If you have not yet studied the inside of the shell go back to "Internal Shell Features" and do so using the right valve.
Muscles
Look at the body of the animal in the left valve. Return the right mantle skirt to its original position and inspect its outer surface. This is the surface that would normally lie adjacent to the inner surface of the right valve. Several muscles originate on the valves and pass through the mantle skirt to their insertion. Some are adductor muscles inserting on the other valve whereas others are pedal muscles inserting on the foot.
Find the large anterior and posterior adductor muscles (Fig 3). You have already seen their scars on the valves. These adduct the valves by bringing them together on the midline thereby closing the gape. No muscles antagonize the adductors, instead the elastic ligament opens the gape and stretches the adductors.
Unionoids also have a pair of pedal protractor muscles to assist in protruding the foot out of the gape and two pairs of pedal retractor muscles to withdraw the foot back into the mantle cavity before the adductor muscles close the gape. The protractors and retractors are antagonists and their origins and insertions must be arranged so they can have opposite actions. Both originate at scars on the inner surface of the valve (Fig 2) and insert on the lateral surface of the foot and visceral mass. Protractor origins (on the shell) are ventral to those of retractors whereas protractor insertions (on soft tissue) are dorsal to those of the retractors.
Near the postero-ventral corner of the anterior adductor find the right pedal protractor muscle (Fig 3). It is a small, but conspicuous, cord of muscle fibers extending obliquely posteriorly and dorsally from its scar on the right valve (Fig 2). Its origin on the valve is ventral to that of the pedal protractor muscle (Fig 2). You can see the muscle by lifting the mantle skirt to reveal the foot. It is usually easy to see and is often pale, either tan or white.
The right anterior pedal retractor muscle is near the pedal protractor but is a little harder to find. Its distal end (origin) is contiguous with the posterior edge of the anterior adductor muscle (Fig 3). The posterior pedal retractor muscle can be seen immediately dorsal to, and contiguous with, the dorsal edge of the posterior adductor muscle. It extends to the dorsal edge of the foot.
The mantle is attached to the inside of the valves by pallial muscles along the periphery of the mantle and by two small inconspicuous muscles extending from the dorsal mantle to the valve near the umbones.
Foot
The foot is a large, laterally compressed complex of muscles with a central hemocoel on the ventral midline of the visceral mass. It will be contracted in both preserved and fresh dissected specimens. In life it is capable of great extension, of which you see no hint in your preserved specimen. The ancestral bivalve foot was adapted for digging in soft sediments and that continues to be true of almost all North American freshwater bivalves, native and introduced, except Dreissena (Fig 12-105). The pedal retractor and protractor muscles originate on the shell and extend to their insertions on the foot.
Mantle Skirt
The two mantle skirts are lateral outfoldings of the mantle, or dorsal body wall of the visceral mass. They extend ventrally on either side of the mantle cavity, visceral mass, and foot and underlie the valves. The right mantle skirt should presently be uppermost in your preparation and should be hiding most of the remaining soft anatomy from view. Move the right mantle skirt dorsally to reveal the mantle cavity, foot, and visceral mass. This will also uncover the left mantle skirt lying intact against the left valve. Avoid disturbing the fragile (in preservative) connection between the left skirt and left valve.
Use the dissecting microscope (8X) and good illumination to examine the ventral margin of the left skirt and note that it consists of four distinct longitudinal folds or ridges extending along its entire length (Fig 4, 12-91). The parallel folds are separated by three parallel grooves. Use fine forceps and needles to demonstrate the presence of four side-by-side folds separated by three grooves. In most bivalves only three folds, inner, middle, and outer, are present but in unionoids one of the original three folds is divided into two folds to give a total of four.
The thick, wide inner mantle fold can be recognized by the small papillae along its free edge. It is the muscular fold containing the radial pallial muscles that extend to the pallial line as well as longitudinal pallial muscles whose fibers parallel the mantle margin (Fig 12-91). It is not involved in secreting the shell. The groove separating this fold from the middle fold is shallow.
The next two folds are separated by the deep periostracal groove. The periostracum originates in this groove and in an intact specimen will still be connected here. New periostracum is secreted by the epithelium of the medial surface of the outer fold, deep in the groove. Look along the edge of the left mantle skirt for places where the skirt remains attached to the shell by a thin, transparent, yellowish-brown, cellophane-like periostracum. The periostracum emerges from the periostracal groove between the outer and middle folds. The attachment between periostracal groove and periostracum is very fragile, and easily destroyed in preserved specimens. It may not be intact in your specimen. The attachment is robust in fresh specimens.
With forceps tug the periostracum out of the periostracal groove without damaging the edge of the skirt. Now, with magnification, examine the edge of the left skirt in a region from which the periostracum has been removed.
The thin middle mantle fold lies close to the outer mantle fold (once the periostracum has been removed) and the two may appear to be a single fold. Use a microneedle to tease the two folds apart and demonstrate the deep periostracal groove between the outer and middle folds. The periostracum is secreted by secretory cells of the outer fold at the bottom of this groove. The middle mantle fold is sensory.
Figure 4. Cross section of the ventral margin of the mantle skirt. Mussel148L.gif
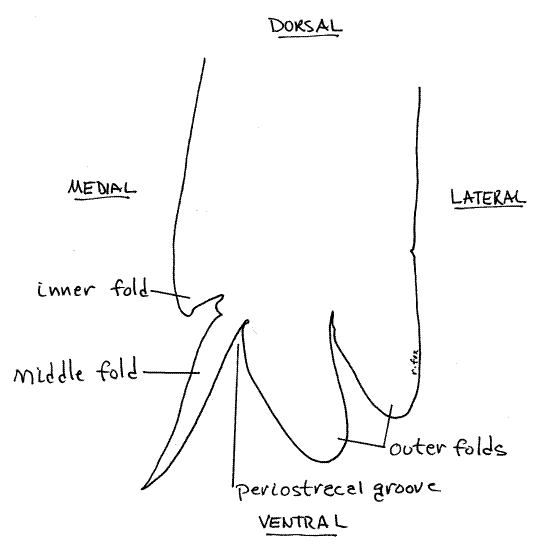
The thick outer mantle fold (the one closest to the shell) is glandular and its outer epithelium secretes the prismatic layer of the shell. In unionoids the outer fold is actually composed of two folds. The entire outer surface of the mantle skirt secretes the lamellar (nacreous) layer of the shell. The mantle folds are not involved in secretion of the lamellar layer.
At some point during the dissection the weakened (by preservative) attachments between muscles and shell in preserved specimens may fail so that the mussel falls out of the left valve. You may take advantage of this, if you wish, to view all aspects of the external anatomy without the interference of a valve. Be sure you are always able to replace the animal in its correct position and orientation in the left valve.
Visceral Mass
The large central part of the body is the visceral mass (Fig 3, 12-90). The foot occupies its median ventral border and the two mantle skirts arise from its dorsal margin. Most of the visceral organs, including the heart, kidney, gut and digestive ceca, and gonads, of a mollusc are contained within the visceral mass. The pigmented tissues of some these organs can be seen through the body wall at present but will be considered in more detail later.
The pericardial cavity occupies the extreme dorsal edge of the visceral mass between the posterior adductor muscle and the umbo. It is covered dorsally by a thin region of the body wall. This body wall is usually opaque in preserved specimens but may be translucent in fresh material. The pericardial cavity is the much reduced bivalve coelom, the chief body cavity of molluscs being the hemocoel. The heart, consisting of two atria and a ventricle, is located in the pericardial cavity, as is a portion of the rectum. (If you are dissecting a living mussel the beating heart may be visible inside the thin body wall and pericardium.) Later in the dissection the pericardium will be opened to reveal its contents.
The brown kidney lies close to the surface of the visceral mass lateral, anterior, and posterior to the pericardial cavity (Fig 12-89B). The greenish digestive cecum (= digestive gland) shows through the body wall of the anterior visceral mass just posterior to the anterior adductor muscle. The yellowish gonad is situated in the central region of the mass dorsal to the foot (Fig 12-89B).
Mantle Cavity
Chambers
The two mantle skirts enclose a large water space known as the mantle cavity. The gills form the roof of this chamber. In life position the mantle cavity is in communication with the external environment via two apertures, the inhalant and exhalant apertures (Fig 12-89A).
On each side of the visceral mass and foot, a gill divides the mantle cavity into a ventral inhalant chamber (= branchial chamber) and a dorsal exhalant chamber (= suprabranchial chamber, epibranchial chamber, cloacal chamber, anal chamber). The gills are the floor of the exhalant chamber and roof of the inhalant chamber. The inhalant chamber is currently visible to you as the large space into which the gills protrude (Fig 3, 12-89A). Most of the exhalant chamber, however, is currently hidden. Looking into the exhalant aperture the space you see is the posterior end of the exhalant chamber. Water enters the inhalant aperture and flows into the inhalant chamber. It then passes through minute ostia in the gills to enter the exhalant chamber from which it exits through the exhalant aperture. The mantle cavity is a space outside the body of the mussel even though parts of it appear to be internal. It contains a current of river water that circulates through the mussel.
Apertures
In unionoids the two mantle margins are largely independent of each other and are not fused except posteriorly where they form two apertures to channel water in and out of the mantle cavity. Together the right and left posterior mantle margins form the ventral inhalant aperture and the dorsal exhalant aperture (Fig 3, 12-106, 12-89). The right and left mantle skirts are fused together dorsal and ventral to the exhalant aperture. The exhalant aperture opens from the exhalant chamber of the mantle cavity to the outside.
The inhalant aperture, although much larger than the exhalant, is not so obvious in a gaping specimen in which the mantle edges do not touch. It is formed by thickened pads on the margins of the right and left mantle edges. When the valves are close together, so are these pads and they then form an opening, the inhalant aperture. Push the right and left posterior mantle edges together recreate the inhalant aperture. The dorsal margin of the inhalant aperture is formed by the fusion of right and left mantle skirts which also forms the ventral margin of the exhalant aperture. In contrast, there is no fusion of tissues to form the ventral margin of the inhalant aperture. The inhalant aperture is equipped with short chemosensory papillae but these will be contracted in preserved specimens. The exhalant aperture has no sensory papillae. The inhalant aperture opens from the outside into the inhalant chamber (= branchial chamber) of the mantle cavity.
By looking into the exhalant aperture you can see the posterior adductor muscle passing transversely across the exhalant chamber. The tubular rectum can be seen on the midline of the posterior adductor muscle. The rectum ends there at the anus, which you can also see by looking in the exhalant aperture.
Look dorsal to the posterior adductor at the junctions of the margins of the right and left mantle skirts. In most unionoids (viz, Ambleminae sF and Unioninae sF but not Margaritiferidae F) there are one or more supra-anal apertures between the two skirts. These openings are auxiliary exhalant apertures dorsal and anterior to the exhalant aperture. Use a blunt probe to demonstrate the continuity of the supra-anal aperture(s) with the exhalant chamber.
Gills
Observe that each gill (there is one on each side) is composed of two half-gills known as demibranchs. A whole gill is a holobranch. Each holobranch is derived from a single bipectinate gill of the ancestral bivalve and thus should still be thought of as a single gill. The mussel has two holobranchs (one right and one left) composed of two demibranchs each for a total of four demibranchs. Each holobranch consists of a lateral demibranch adjacent to the mantle skirt, and a medial demibranch adjacent to the visceral mass and foot (Fig 3, 12-90). The holobranch is attached to the roof of the mantle cavity by a longitudinal central axis. The central axis lies between the two demibranchs.
" Use a pair of scissors to remove the right mantle skirt with a longitudinal incision between the dorsal margin of the right lateral demibranch and the right mantle skirt. Start the incision immediately ventral to the posterior adductor muscle and avoid cutting into the region of the apertures. Extend the incision anteriorly and then dorsal to the labial palps and remove the skirt. This incision will open the exhalant chamber but the gill and labial palp will remain with the body. Modify the incision if necessary so that the exhalant chamber is open along the entire length of the gill. Do not open the pericardial cavity at this time.
Bivalve gills are composed of numerous slender gill filaments joined together to form sheets. Use 30X of the dissecting microscope to look at the surface of the lateral demibranch and you will see the very fine parallel gill filaments (Fig 12-96D). Each filament begins at the central axis, drops down into the inhalant chamber then reverses direction sharply and climbs back up to the roof of the chamber where it attaches beside (lateral or medial) to the central axis. Each filament is attached to the filament anterior to it and the one posterior to it. Collectively all the filaments are joined together form a sheet, or lamella. Each demibranch is composed of two lamellae (Fig 12-96D). One, the descending lamella is composed of the filaments that drop down from the central axis (Fig 12-90). The other, the ascending lamella, is composed of the same filaments on their way back up to the top of the inhalant chamber.
Look at the lateral demibranch of the right gill. It should be facing you. The surface you see is the ascending lamella of the lateral demibranch. Lift the demibranch and look at its other side. This is the descending lamella of the lateral demibranch. Look at its dorsal edge to see the central axis. With the lateral demibranch held up and out of the way you are looking at the descending lamella of the medial demibranch. It arises at the central axis. Finally, lift the medial demibranch and look at its medial surface. This heretofore hidden surface is the ascending lamella of the medial demibranch. Got it? Two holobranchs, two central axes, four demibranchs, eight lamellae in one mussel.
Most bivalves, unionoids included, use their gills for suspension feeding as well as gas exchange. Both functions require that water (with dissolved oxygen and suspended food particles) enter the inhalant chamber, pass through tiny openings in the gill lamellae (between the filaments) into the exhalant chamber and then out the exhalant aperture. Oxygen and food particles are removed as the water passes through the lamellae. Adjacent filaments are held together by interfilamentary junctions but these are not unbroken continuous connections (Fig 12-96D). Gaps in the junctions are known as ostia and provide a route for water to flow through the lamellae.
" With fine scissors cut a 2x2 mm square of the ascending lamella and rinse both sides of it with a vigorous stream of water from a squeeze bottle. Make a wetmount and examine it with 400X of the compound microscope. Most of what you see will be filaments. Tissue interfilamentary junctions holding the filaments together will also be visible. The clear areas between the filaments, where the junctions are interrupted are ostia. Focus carefully on the edges of the filaments to see the lateral cilia extending from the filament into the ostia (Fig 12-99B, 12-98). These are the cilia that generate the feeding/respiratory current through the system. Other cilia, known as frontal cilia, are on the inhalant surfaces of the lamellae and are responsible for moving food particles and mucus over the gill. Frontal cilia may be difficult to see in this preparation.
Look again at the surface of the ascending lamella of the lateral right demibranch. Large evenly spaced ridges extend across the demibranch parallel to the filaments. These are tissueinterlamellar junctions responsible for holding the ascending and descending lamellae of the demibranch together. The interlamellar junctions divide the exhalant chamber into vertical water tubes (Fig 3, 12-98C, D) that arise in the demibranch and empty into the exhalant chamber. We have seen that the lamella appears finely corrugated because it is composed of filaments but coarser corrugations are also present and these are the water tubes. The demibranch was opened in the vicinity of the exhalant aperture earlier when you cut away the right mantle skirt. Find the openings to the water tubes inside the lateral demibranch (Fig 3). Note that the tubes open into the exhalant chamber.
The kidneys and gonad connect via through separate ducts with openings in the anterior end of each exhalant chamber. Both are small slits of which the nephridiopore is slightly lateral to the gonopore.
Female unionoids retain their eggs and brood them in the water tubes. Note if eggs, embryos, or larvae are present and if so return later during your study of reproduction to make a wetmount of them.
Labial Palps
A large, leaflike labial palp is located on each side of the visceral mass and gill at the anterior end of the mantle cavity (Fig 3, 12-112A). Each palp resembles a small gill and like the gill is bilobed, being composed of two similar, demibranch-like half palps. The lateral half-palp is associated with the lateral demibranch of the whereas the medial half-palp corresponds with the medial demibranch.
Figure 5. Ventral view of the anterior end of the foot and visceral mass. The palps are opened to reveal the sorting fields. Most of the ciliated ridges of the left palp have been omitted. Mussel150L.gif
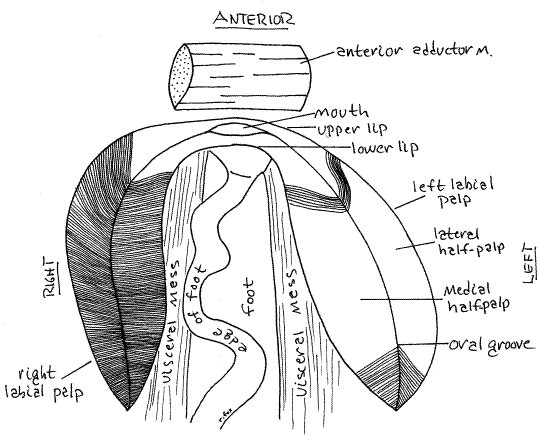
Find the right labial palp and lift the lateral half-palp to expose the medial half-palp (Fig 5). Particles and mucus are transported by ciliary currents from each demibranch to the associated half-palp for sorting. Examine the now-exposed surfaces of the half-palps with magnification to see that they are composed of an array of ciliated ridges and grooves. These form a ciliary sorting field across which a string of mucus and food particles from the gills passes on its way to the mouth. While crossing the palpal sorting field mineral particles are imperfectly separated from organic food particles. The mineral particles drop off the edges of the palp into the inhalant chamber as pseudofeces whereas food particles continue on to the mouth. In life the ciliated, ridged surfaces of the two halves of the palp face each other and the sorting field is not visible from the outside. In the crease where the two half-palps join is a ciliated oral groovethat goes to the mouth (Fig 12-94D). Mineral particles are transported laterally, away from this groove and food particles move along it.
If the mussel is still in the left valve, remove it and set the valve aside. Hold the mussel so you can view the anterior end of the visceral mass and foot with magnification (Fig 5, 12-100). Open the right palp so the sorting fields of both half-palps are completely exposed. Manipulate the mussel so you can trace the anterior ends of each half palp anteriorly toward the midline. The anterior end of the lateral half-palp tapers to a narrow ridge of tissue that extends anteriorly to cross the midline where it joins the similar anterior extension of the left lateral half-palp. The anterior extension of the right medial half-palp similarly joins with the left medial half-palp. These two ridges cross the anterior midline of the visceral mass immediately dorsal to the foot and ventral to the anterior adductor muscle. Located between them is the large, open mouth (Fig 5, 12-89B). The ridge connecting the lateral half-palps is dorsal to the mouth and forms the upper lip. Thelower lip connects the two medial half-palps below the mouth. The mouth is much easier to see in unionoids than in most other bivalves.
Internal Anatomy
Nervous System
The central nervous system is typical of bivalves and consists of paired ganglia connected by commissures and connectives (Fig 8, 12-119). In life the ganglia contain neuroglobin which, if it has not faded in preservative, imparts a rusty orange, brownish, or yellow color and makes them easier to locate.
" The large visceral ganglion is easily found making it a good starting point for dissection of the CNS. The paired visceral ganglia are fused together on the midline to form what appears to be a single ganglion. It is on the ventral margin of the posterior adductor muscle and may or may not be visible through the thin epithelium covering the midline of the ventral surface of this muscle. Remove this epithelium to expose the ganglion. Three major pairs of nerves exit the ganglion. A pair of conspicuous cerebrovisceral connectives (= visceral nerves) connect it with the cerebral ganglia. The connectives can be traced anteriorly into the visceral mass for a short distance but anteriorly they are embedded in the mass and cannot be seen without careful dissection. A pair of large posterior pallial nerves from the visceral ganglia serve the posterior mantle margin (Fig 12-119, 12-91). A pair of large branchial nerves from the visceral ganglion extend to the gills. The heart is served by a pair of small nerves from the visceral ganglion.
The smaller cerebral ganglia are situated anteriorly, one on each side of the mouth (Fig 8). They are more difficult to locate than the visceral ganglia. Find the mouth and the anterior pedal protractor muscle and pedal protractor muscle where they emerge from the visceral mass. The lips of the labial palps pass over this area which is separated from the lateral corners of the mouth by a few millimeters. The ganglia are not immediately adjacent to the mouth. Carefully remove the thin body wall from the area just described to reveal the ganglion. The pleural ganglia are fused with the cerebral ganglia and cannot be distinguished from them in gross anatomy.
The two cerebral ganglia are connected by the slender cerebral commissure which arches dorsally over the mouth. A cerebropedal connective extends from each half of the cerebral ganglion to the pedal ganglion. An anterior pallial nerve extends longitudinally along the anterior mantle margin after arising from the cerebral ganglia. Each half of the cerebral ganglion sends a nerve to the anterior adductor muscle.
The two pedal ganglia are fused on the midline in the dorsal edge of the foot, embedded in the foot muscles (Fig 8). They can be exposed only through a careful dissection beyond the scope of this exercise. They connect with the cerebral ganglia by the cerebropedal connectives mentioned above. The short pedal commissure is embedded between the two contiguous pedal ganglia and cannot be seen in gross view. Together these elements (cerebral ganglia, cerebral commissure, cerebropedal connectives, pedal ganglia, and pedal commissure) form a nerve ring around the anterior gut (esophagus). Motor nerves from the pedal ganglion the muscles of the foot. A pair of spherical statocysts is located lateral to each pedal ganglion.
The peripheral nervous system consists of sensory and motor nerves extending to and from the above ganglia. The most important have been mentioned.
Hemal System
The bivalve hemal system consists of a heart, arteries, veins, and a hemocoel consisting of large, blood-filled sinuses. The blood is colorless. The heart is located in the dorsal pericardial cavity mentioned earlier, and is easily demonstrated. The remainder of the hemal system is difficult to see in these specimens.
" Relocate the thin dorsal body wall on the dorsal midline just anterior to the posterior adductor muscle. This part of the body wall covers the pericardial cavity (Fig 3, 12-89B). Use fine scissors to make a longitudinal incision through this thin body wall to open the pericardial cavity. The incision should extend from the posterior adductor muscle anteriorly to near the posterior edge of the umbo. Be careful that you cut only through the thin body wall and that you do not damage the organs within the pericardial cavity.
The relatively large space this incision exposes is the pericardial cavity lined by a peritoneum known as the pericardium. It is a coelomic space and contains the heart and rectum. A large tube, the rectum, enters the cavity anteriorly, extends the longitudinally for length of the cavity, and then exits posteriorly to end at the anus on the posterior margin of the posterior adductor muscle (Fig 6, 12-89B). The rectum passes through the center of the heart ventricle.
The heart consists of a single medial ventricle and two lateral atria (= auricles), one on either side of the ventricle. The atria and ventricle are both contractile.
The large and conspicuous ventricle is wrapped around the rectum and is easy to find, although its relationship with the rectum may surprise you. Blood exits the ventricle via an unpaired median anterior aorta lying on the dorsal surface of the rectum, and an unpaired median posterior aorta on the ventral surface of the rectum. The aortae are difficult to demonstrate. The anterior aorta supplies the visceral mass, anterior adductor muscle, gonad, foot, anterior mantle, and kidney. The posterior aorta supplies the posterior adductor muscle, pericardium, rectum, and the posterior mantle. The venous return consists of veins and sinuses ultimately draining into the vena cava, a large sinus ventral to the pericardial cavity and between the two kidneys. Blood from the vena cava goes to the gills via the afferent branchial vessels where. Oxygenated blood leaves the gills via the efferent branchial vessels to the atria. The atria drain into the ventricle.
Gently pull the ventricle and rectum to the side, away from the body wall while watching under magnification. This will enhance your view of a thin, triangular, membranous atriumconnecting the side of the ventricle with the membranous efferent branchial vessel from the right (or left) gill.
Figure 6. Dorsal view of the opened pericardial cavity. Mussel151L.gif
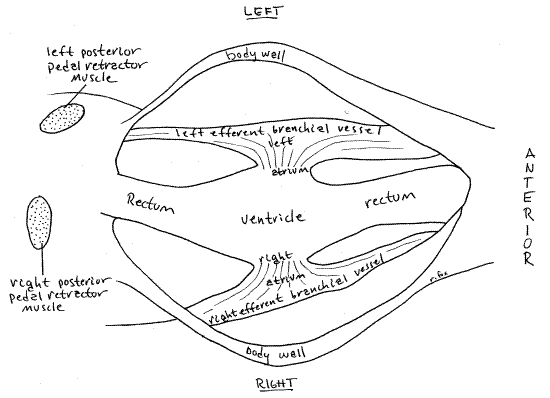
" Use fine scissors to open the right side of the ventricle with a longitudinal incision along the entire ventricle. This will open the ventricular lumen for study. Observe the thick muscular walls of the ventricle. Notice the conspicuous tubular rectum passing longitudinally through the lumen. Look on the right side of the ventricle for an atrioventricular aperturebetween the atria and ventricle. The aperture is guarded by an atrioventricular valve consisting of two thin, membranous, longitudinal folds of tissue arranged like lips above and below the aperture. Although large, these lips can be difficult to distinguish from the muscular ventricular walls and from each other. Use fine forceps and a microneedle to probe the tissues and demonstrate the lips. The two lips form a one-way valve that prevents the backflow of blood from the ventricle to the atria. You can see, by examining the configuration of the lips, how back pressure on the membranes would close the aperture.
Contractions of the atria propel blood through the atrioventricular aperture into the ventricle. Subsequent contraction of the ventricle closes the atrioventricular valve and forces blood into the anterior and posterior aortae.
Excretory System
The bivalve excretory system consists of a pair or large, elaborate metanephridia, or kidneys, draining the pericardial cavity to the exhalant chamber (Fig 12-89, 12-118). Remember that the pericardial cavity is a coelom. Each kidney is an elaborate tube extending from the nephrostome, opening from the pericardial cavity, to the nephridiopore, opening into the exhalant chamber. A special elaboration of the atrial wall, equipped with podocytes, is specialized for ultrafiltering the blood into the pericardial cavity to form the primary urine. The primary urine enters a nephrostome and passes through the kidney to the nephridiopore. During passage through the kidney lumen the primary urine (ultrafiltrate) is modified, chiefly by reclamation of solutes, and the resulting final urine, mostly water, is released from the nephridiopore into the exhalant chamber. The role of the kidneys in freshwater bivalves, living as they do in a hyposmotic environment, is chiefly osmoregulatory. The kidneys constantly pump excess water out of the tissues and back into the environment. Ammonia, the chief end product of nitrogen metabolism, is lost by diffusion across the mantle and gill surfaces.
" Each of the two kidneys (= metanephridium, organ of Bojanus, renal organ) is a large sac located ventral, lateral, and posterior to the pericardial cavity (Fig 7). With fine scissors make a longitudinal cut through the body wall along the dorsal margin of the right gill and ventral to the pericardium.
Figure 7. Dissected unionoid mussel viewed from the right side showing the pericardial cavity and kidney. The gill and visceral mass are drawn as if transparent. Mussel152L.gif
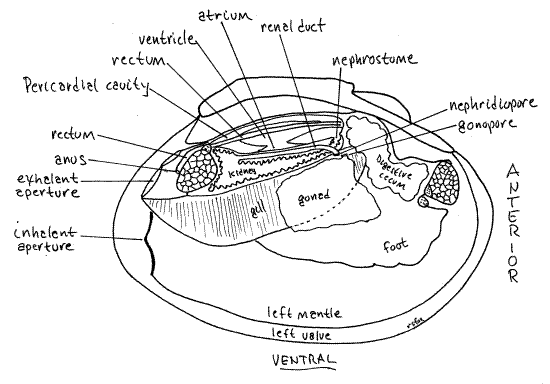
The kidney is recurved on itself and open at both ends (Fig 7, 12-120C, 12-118). Proximally it is a large spacious glandular region that opens from the anterior pericardium via the nephrostome. The distal, downstream end of the kidney is a non-glandular renal duct that opens into the anterior exhalant chamber via the nephridiopore. The ventral wall of the pericardial cavity is adjacent to the dorsal wall of the glandular portion of the kidney. If your incision was deep enough it will have opened the glandular kidney and revealed its lumen. The walls have a glandular appearance and consist of dark brownish gray tissue. The nephridiopore is a slit-like opening at about the level of the middle of the foot but it is not easy to demonstrate. The gonopore, either male or female, is nearby, slightly medial to the nephridiopore.
The two pericardial glands (= Keber’s organs) are elaborations of the pericardial peritoneum equipped with abundant podocytes where ultrafiltration occurs (Fig 12-118). These brown organs can be seen through the body wall at the anterior end of the pericardial cavity. They receive blood from the efferent branchial vessel and ultrafilter it into the pericardial cavity to form the primary urine.
Reproductive System
Anatomy
Unionoids are gonochoristic with external cross fertilization. Some sexual dimorphism is present but it is usually not conspicuous (the shell of females of some species is more inflated than males to provide the space for brooding). The reproductive system consists of a single gonad, either ovary or testis, and two short gonoducts. The gonad, although a derivative of the coelom, is independent of the pericardial cavity. It does not share ducts with the kidney (Fig 12-120C). It arises in bivalve evolution through the fusion of an ancestral pair of gonads. The two gonoducts, right and left, emerging from a single gonad, reflect this double origin. The gonad is large and occupies the space in the visceral mass extending ventrally from the kidney to the dorsal edge of the foot (Fig 8, 12-89B). Each gonoduct empties into the anterior exhalant chamber via a gonopore.
Figure 8. Dissected Actinonaias showing the gonad, digestive system, and ganglia. The star-shaped visceral ganglion is shown a little larger than life size. A = anterior, g = ganglion, m = muscle. Mussel99L.gif
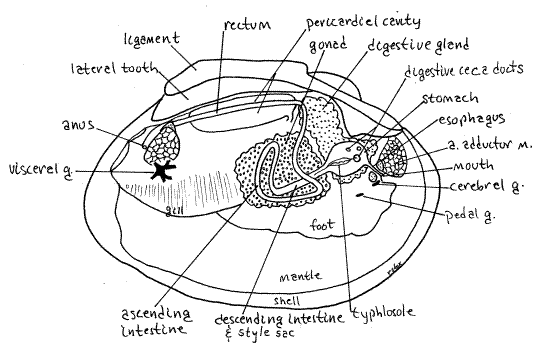
" The shapeless gonad can be exposed by cutting away the thin body wall from the side of the ventral visceral mass at the top of the foot. It fills the space in the ventral visceral mass and is dorsal to the muscular foot.
Life Cycle
Females brood eggs and embryos to the glochidia larva stage in the water tubes (Fig 12-121C, D). Females shed eggs into exhalant chamber from which they move into the water tubes of some or all demibranchs. In some (viz. Amblema, Quadrula, Fusconaia, Gonidea, Tritogonia, Plectomerus, Quincuncina, and Megalonais) both demibranchs on each side are used as brood chambers and are visibly swollen in brooding females but in the remaining Unionidae, including Actinonaias, Lampsilis, and Anodonta, only the lateral demibranchs are so used.
Sperm are shed into the river (or lake) from the exhalant aperture of a male individual. If caught in the inhalant flow of a female mussel, they pass through the ostia to enter the water tubes of the demibranchs. The waiting eggs are fertilized and retained in the water tubes where development begins and continues to the glochidia larva stage.
Glochidium Larva
Females release glochidia larvae through the exhalant aperture (Fig 12-121E). Glochidia are modified veliger larvae and are parasitic, requiring a fish host before metamorphosing into a juvenile mussel capable of independent existence. Glochidia parasitize and feed on a fish host before dropping off into the sediment to metamorphose into a juvenile mussel.
> b. Inspect the water tubes for the presence of glochidia larvae. If present, make a wetmount with a wax supported coverslip and study it with the compound microscope. Glochidia are derived bivalve larvae with a pair of valves, adductor muscle, sensory bristles, and a larval thread (Fig 12-121C). Species that parasitize the skin of their fish hosts also have a conspicuoushook at the ventral border of each valve (Fig 12-121C). Species that attach to the gills lack the hooks. <
Digestive System
The digestive system consists of mouth, esophagus, stomach with digestive ceca, intestine, and anus. Remove the mussel from both valves if you have not already done so. The largemouth is on the midline at the anterior end of the visceral mass flanked by a labial palp on each side (Fig 5, 8, 12-89B). Hold the mussel with one hand and examine the anterior midline of the visceral mass to find the mouth.
The mouth opens into the short esophagus which extends posteriorly to the stomach. Insert a blunt probe into the mouth and look into the esophagus while holding the mussel on the stage of the dissecting microscope.
" With the mussel in a small dissecting pan of water on the stage of the dissecting microscope use fine forceps to dissect away the thin body wall posteriorly from the right side of the mouth. You may already have done some of this to expose the right cerebral ganglion. Removal of the body wall will expose the esophagus, which is a short but wide, very flat, thin-walled tube about the width of the mouth (Fig 8). With fine scissors open the esophagus posteriorly to the stomach. The esophagus is lined by a ciliated epithelium. Further dissection of the digestive system is destructive and should not be attempted until all other organ systems have been studied.
Extend the esophageal incision posteriorly to open the anterior stomach. Initially you need cut only the thin gut wall but to open the posterior stomach you must cut through a thick layer of the wall of the visceral mass.
The stomach is surrounded by the two greenish digestive ceca which can usually be seen, without dissection, through the surface of the visceral mass posterior to the anterior adductor muscle (Fig 8). The ceca are diverticula of the stomach to which they remain connected by ducts opening from the stomach walls (Fig 12-103B).
The stomach is a large chamber in the anterior dorsal visceral mass (Fig 8, 12-102). With magnification examine the anterior folded walls of the stomach. Anteriorly are four openings to the digestive ceca. The largest opening is low on the left wall, another is to the right of it, and two others on the dorsal wall. All these openings are in the anterior half of the stomach. Insert one blade of your fine scissors one of the apertures and open the duct with which it connects. This duct will soon enter a greenish digestive cecum. The ceca walls are elaborated to form abundant acini, or pouches, lined with secretory and absorptive epithelium (Fig 12-103B). Absorption and most digestion, both intra- and extracellular takes place in the ceca. Indigestible particles are returned to the stomach. Some extracellular digestion takes place in the stomach.
Much of the wall of the stomach bears the fine but conspicuous, parallel, ciliated ridges and grooves of sorting fields whose function is to separate incoming particles and send organic particles to the digestive ceca and mineral particles to the intestine.
On the ventral stomach wall is the large protuberant ciliated typhlosole whose role is shunt mineral particles wasted from the digestive ceca to the intestine. Note that a slender ridge continuous with the larger portion of the typhlosole exits the large ventral aperture.
Posteriorly the intestine and style sac share a common bilobed aperture from the stomach. This aperture is partially divided, by two ridges, into the intestine on one side and the style sac on the other. Notice that the typhlosole enters this opening (Fig 8, 12-102). Most material entering the intestine is indigestible mineral particles and the chief function of the intestine is feces formation and storage.
The ciliated, secretory epithelium lining the style sac secretes and rotates an elongate, flexible, pellucid rod, the crystalline style, which extends out of the style sac into the lumen of the stomach. The distal (stomach) end of the style rubs against a chitinous plate, the gastric shield, in the wall of the stomach. The style is composed of digestive enzymes secreted by the sac epithelium and is present only in individuals that are feeding or have recently fed. It is resorbed in starved individuals. Consequently it may not be present in your specimen. It is large an unmistakable when present.
" Follow the lumen of the style sac and intestine into the gonad ventral to the stomach. The style sac and intestine extend, side by side with continuous lumina, into the visceral mass where they are surrounded by the gonad. Here the style sac eventually reaches a dead end and stops but the intestine continues on making several loops until it eventually reaches the anus. The intestine is divided into three regions but its path through the visceral mass is difficult to follow. Use your scissors to trace the intestine lumen as far as you can. Beginning in the posterior stomach insert one blade of the scissors into the opening of the intestine and style sac and cut completely through the thick wall of the visceral mass. Most of this wall is gonad. You will cut through digestive ceca (greenish) and gonad (yellowish) as you trace the gut.
The descending intestine exits the posterio-ventral end of the stomach and its lumen is beside and continuous with that of the style sac for the length of the sac (Fig 8, 12-89B, 12-103A). It and the sac are a straight bilobed tube extending into the gonad in the ventral visceral mass.
Deep within the visceral mass, the style sac ends and the intestine continues as the ascending intestine, or middle limb of the intestine. This second of three regions of the intestine loops through the gonad and then extends dorsally to exit the gonad. The walls of the middle intestine are thin, making this limb difficult to trace in its wanderings.
Having exited the gonad, the gut extends posteriorly as the rectum (Fig 8, 12-89B). The rectum, which you have already seen, enters the pericardial cavity, passes through the ventricle, exits the cavity, and curves dorsally over the posterior adductor muscle to end at the anus on the posterior side of the muscle. Insert a teasing needle into the slit-shaped anus to demonstrate its presence.
" The wall of the rectum is folded to form a large typhlosole that occupies most of the lumen. Relocate the rectum where it enters the anterior end of the pericardial cavity and trace it through the ventricle. With a longitudinal incision open the rectum and find the typhlosole.
References
Alderman JM, Ratcliffe JA, McDougal LA. North Carolina Freshwater Mussels; Wildlife Species and Conservation. www.ncwildlife.org/pg07_WildlifeSpeciesCon/pg7b1a.htm
Bogan A . Workbook and Key to the freshwater bivalves of North Carolina
http://www.naturalsciences.org/research/inverts/bogan.html
Brooks WK. 1890. Handbook of invertebrate zoology for the laboratory and seaside work. Bradlee, Whidden Pub, Boston. 392pp.
Bullough WS. 1958. Practical invertebrate anatomy, 2 nd ed. MacMillan, London. 483 pp.
Burch JB. 1973. Freshwater unionacean clams (Mollusca: Pelecypoda) of North America. US Env. Protection Agency, Biota of Freshwater Ecosystems, Project 18050ELD, Contract 14-12-894. Govt. Printing Office stock number 5501-00488. 176pp.
Campbell DC, Serb JM, Buhay JE, Roe KJ, Minton RL, Lydeard C. 2005. Phylogeny of North American amblemines (Bivalvia, Unionoida): prodigious polyphyly proves pervasive across genera. Invertebrate Biology 124(2):131-164.
Cummings K,Bogan A,Watters GT,Mayer CA . Freshwater Mollusk Bibliography
http://ellipse.inhs.uiuc.edu/mollusk/
Freeman WH, Bracegirdle B. 1971. An Atlas of invertebrate structure. Heinemann Educational Books, London. 129 pp.
Johnson RI . 1970. The systematics and zoogeography of the Unionidae (Mollusca: Bivalvia) of the southern Atlantic Slope Region. Harvard Univ, Bull. Mus. Comparative Zoology 140(6):263-447.
McMahon RF. 2001. Mollusca: Bivalvia, pp.331-430 in Thorp JH, Covich AP (eds.), Ecology and classification of North American freshwater invertebrates, 2 nd ed. Academic Press, San Diego. 1056pp.
Morse MP and Zardus JD. 1997. Bivalvia, pp. 7-118 in Harrison FW, Kohn AJ, Microscopic anatomy of invertebrates 6A, Mollusca II. Wiley-Liss, New York. 414 pp.
Parmalee PW, Bogan AE. 1998. The freshwater mussels of Tennessee. Univ. Tennessee Press, Knoxville. 328 pp
Pennak RW. 1989. Fresh-water invertebrates of the United States, 3 rd ed. Wiley, New York.
Pierce ME. 1950. Fresh-water clams or mussels. Pp. 334-336 (with figure on p.332) in Brown FA. Selected invertebrate types. Wiley, New York. 597 pp.
Ruppert EE, Fox RS, Barnes RB. 2004. Invertebrate Zoology, A functional evolutionary approach, 7 th ed. Brooks Cole Thomson, Belmont CA. 963 pp.
Simpson GB . 1884. Anatomy and physiology of Anodonta fluviatilis. Rept. New York State Museum 35:169-191, pls 3-13. Detailed and well illustrated.
Strayer DL, Jirka KJ . 1997. The pearly mussels of New York State. New York State Museum Mem. 26:1-113, pls 1-26.
Williams JD, Neves RJ. Freshwater mussels: A neglected and declining aquatic resource.
http://biology.usgs.gov/s+t/noframe/f076.htm.
Williams JD, Warren MI, Cummings KS, Harris JL, Neves RJ . 1993. Conservation status of the freshwater mussels of the United States and Canada. Fisheries 18(9):6-22.
Supplies
Preserved (or fresh) unionoid (pearly) freshwater mussel
Empty shell consisting of right and left valves
Dissecting pan
Dissecting microscope
Dissecting set
Broken piece of the ventral edge of a valve