Invertebrate Anatomy OnLine
Protozoa ©
9jul2006
Copyright 2001 by
Richard Fox
Lander University
Preface
This is one of many exercises available from Invertebrate Anatomy OnLine , an Internet laboratory manual for courses in Invertebrate Zoology. Additional exercises can be accessed by clicking on the links to the left. A glossary and chapters on supplies and laboratory techniques are also available through this link. Terminology and phylogeny used in these exercises correspond to usage in the Invertebrate Zoology, a Functional Evolutionary Approach by Ruppert, Fox, and Barnes (2004). Hyphenated figure callouts refer to figures in the textbook. Callouts that are not hyphenated refer to figures embedded in the exercise. The glossary includes terms from this textbook as well as the laboratory exercises.
Introduction
“ Protozoa” is an informal collective term used in reference to a polyphyletic assemblage of animal-like protists. Their more plant-like counterparts are usually known as algae. Protozoa includes several taxa of organisms that may have little in common with each other and that probably are not closely related evolutionarily. Most are motile, most are unicellular (or colonial), and are either heterotrophic or have heterotrophic ancestors. Many, such as euglenoids and dinoflagellates, are derived from heterotrophic eukaryotic ancestors that established obligate associations with photosynthetic eukaryotic endosymbionts. Refer to Fig 3-37* of Ruppert, Fox, and Barnes (2004) for a cladogram showing the evolutionary relationships of the major protozoan taxa.
Protozoa includes a startling diversity with about 92,000 described species. They occur in all habitats including marine, freshwater, and terrestrial, including soil. About 25% participate in symbiotic associations and many are the causative agents of important human diseases including malaria, the most deadly human parasitic disease.
Most protozoans are enclosed by a skeletal structure known as the pellicle consisting of the plasma membrane and underlying cytoskeleton. The plasma membrane is the outer surface and its associated cytoskeleton may consist of additional membranes, microtubules, microfilaments, or plates of cellulose or protein. In some, flattened vesicles known as alveoli contribute to the pellicle. The pellicle maintains the shape of the cell. Sometimes there is a secreted, extracellular, non-living test (= shell, theca, lorica).
Much of the cytoskeleton is deeper and is either not associated with the pellicle or only indirectly so. The cytoplasm is typically divided into a thin outer ectoplasm and an inner endoplasm. Protozoans often bear cilia or flagella with the 9x2+2 axoneme and 9x3+0 basal body microtubular structure typical of eukaryotes. Osmoregulation in freshwater protozoans is accomplished by contractile vacuoles that pump a hyposmotic urine from the cytoplasm back into the environment. This counters the osmotic influx of water across the plasma membrane. In many protozoans the contractile vacuole is a conspicuous feature of the cell.
Protozoans reproduce sexually and asexually. Cell division usually involves a closed spindle which, unlike the open spindle of multicellular animals, is constructed inside the intact nuclear envelope.
The organisms included in this chapter are chosen to be representative of the major protozoan taxa. Whenever possible species have been selected that are readily and inexpensively available alive, although the exercises do not require living material and prepared slides can be used if desired. The chapter contains more species than can be covered in a single 3-hour laboratory session.
Euglena
Euglenozoa P, Euglenoidea C
Euglenoids are biflagellated unicells, about a third of which are pigmented and photosynthetic. The taxon includes about 1000 described species, most of which inhabit fresh water. The cells are often spindle-shaped. Clonal reproduction occurs by longitudinal fission (Fig 3-6). Mitosis occurs without a spindle and there is apparently no sexual reproduction.
Euglenoids arose as heterotrophs, some of which secondarily acquired photosynthetic eukaryotic symbionts (chlorophytes, green algae) which have come to function as chloroplasts. The chloroplasts contain chlorophyll a and b and are now thought to have arisen from an ancient endosymbiotic association of green algal cells with an heterotrophic and colorless, heterotrophic euglenoid ancestor. About two thirds of euglenozoans are colorless heterotrophs such as Peranema, and the rest are green autotrophs, such as Euglena, Lepocinclis, and Phacus.
Euglenoids are found in many freshwater habitats and are most abundant in those, such as farm ponds or drainage ditches, that receive animal wastes.
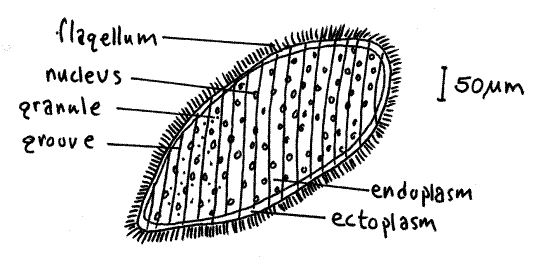
1. Prepare a wet mount of living Euglena using Protoslo. Use a prepared slide if living cultures are not available. Use an absorbent tissue applied to one edge of the coverslip to remove a little water so as to slow the animals a bit (but don't immobilize them yet). Using 100X then 400X, find and study one individual. Observe the movements of the organisms. Note the characteristic fusiform shape of the organism (Fig 1, 3-5A). Do you see cells with other shapes?
Reduce the light intensity with the iris diaphragm and look for a long locomotory flagellum (= primary flagellum) at the anterior end. There are actually two flagella but one, the accessory flagellum, is very short and will not be seen. (If available, use phase contrast and/or dark field to help visualize the locomotory flagellum.) The locomotory flagellum undulates to provide the propulsive force for locomotion. Does the flagellum pull or push the organism through the water?
Remove more water from the preparation so the cells are caught between slide and coverslip and immobilized. Be careful that you do not remove so much water that you crush or seriously distort the cells. Look at several cells as necessary to find all the structures described below.
The outer cytoplasm, or ectoplasm, of Euglena is elaborated to form a thin, semi- rigid but flexible pellicle that holds the cell in its characteristic shape. The pellicle includes the cell membrane, plus an underlying layer of interlocking proteinaceous strips. There is no cellulose.
Figure 1. Euglena. Protozoa142L.gif
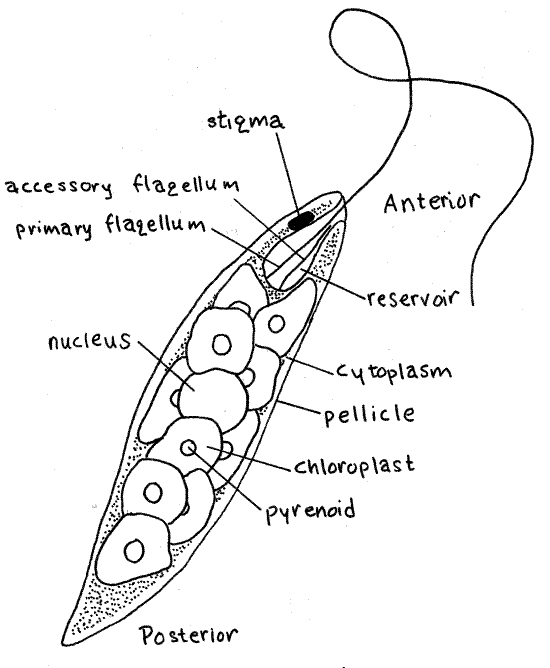
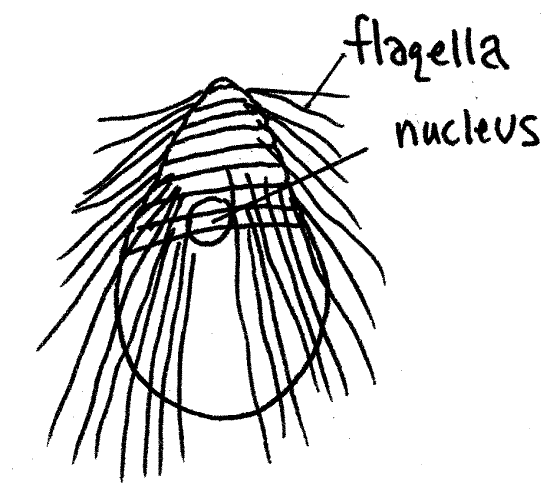
The primary flagellum emerges from an anterior invagination, the reservoir, which connects to the outside by a narrow canal (Fig 1). The transparent reservoir is usually easy to see in immobilized specimens. Both flagella arise from basal bodies in the cytoplasm at the base of the reservoir. The second flagellum is rudimentary and does not emerge from the reservoir.
There is a small, red pigment eyespot, or stigma, located on one side of the reservoir. It is associated with a swollen, light-sensitive region at the base of the locomotory flagellum.
During your work with living Euglena, you may see individuals abandon the flagellar mode of locomotion and begin moving in a curious "euglenoid motion" involving changes in the pellicle and shape of the cell. A bulge moves from one end of the cell to the other. This is likely to occur when the organism is stressed.
A small and inconspicuous contractile vacuole is located to one side of the reservoir, into which it discharges (Fig 3-5A). Its function is osmoregulatory, ridding the cell of excess water. Under ordinary conditions, the vacuole fills and empties about twice a minute but the rate ultimately depends on the osmolarity of the environment.
Note the green color of the cell. Although it may appear otherwise, the color is not distributed evenly through the cytoplasm, a fact that careful observation will confirm. Instead, it is contained in chloroplasts as expected of eukaryotic cells. In Euglena, photosynthate is synthesized, as the polysaccharide paramylon, in pyrenoids associated with the chloroplasts (Fig 1, 3-5A) and stored as granules in the cytoplasm.
The shape, size, number, and arrangement of chloroplasts in Euglena is characteristic of the species. Of the two commonly used laboratory species, the chloroplasts of E. viridis are long, narrow, and usually arranged in a star or sunburst-like radiating pattern whereas the disk-shaped chloroplasts of E. gracilis are scattered randomly through the cytoplasm (Fig 1, 3-5A). In Euglena graclis there is a pyrenoid in the center of each chloroplast. Each pyrenoid is covered by paramylon deposits and paramylon granules are scattered throughout the cytoplasm.
A spherical, grayish nucleus is located near the center of the cell but is difficult to see among the chloroplasts. Its position can sometimes be inferred from the deformation of the surrounding chloroplasts. The nucleus is usually easier to see if some water has been removed from the slide so the cells are flattened slightly.
1a. Add a drop of iodine-potassium iodide (IKI) or Lugol’s solution to the edge of the coverslip and draw it under. This will kill the cells. Study the cells in the presence of iodine. The flagellum should be more visible now. Other features which were formerly difficult to see may now be more apparent. Look again for the nucleus.
References
Beutow (1968a, 1968b), Grell (1973), Leedale (1967), Pringsheim (1956), Sleigh (1989)
Trypanosoma
Euglenozoa P, Kinetoplastida C, Trypanosomatidae F
Kinetoplastids are all heterotrophic and most are parasitic. Common possession of a stiffening paraxial rod in the flagellum unites them with euglenoids. Unique to kinetoplastids is the kinetoplast, a large clump of DNA located at one end of an enormously long (compared to the length of the cell) mitochondrion.
Trypanosomes are small, parasitic kinetoplastids with one flagellum and an undulating membrane. In vertebrate hosts they are blood parasites causing a number of maladies including kala-azar (Fig 3-7A), sleeping sickness, and Chagas’ disease. The intermediate hosts are insects (Fig 3-7B). These diseases, and others, are caused by various members of the genera Leishmania and Trypanosoma . Trypanosoma lewisi, a parasite of rats, is often used in teaching laboratories.
2. This exercise is written for use with commercially prepared slides of smears of infected rat blood but could also be used with living trypanosomes if they are available.
Use 100X to find trypanosomes on your smear and then focus on one with 400X. The tiny trypanosomes are considerably smaller than the red blood cells on the slide. Commercial preparations are usually stained with Wright's stain and if so, the trypanosomes will be blue. You will need the oil immersion lens to examine these small cells.
To use the oil immersion lens, focus on the trypanosome with the high dry lens (40X) and be sure the organism of interest is centered in the middle of the field. Rotate the nosepiece halfway to the oil immersion (100X) objective. Place a drop of immersion oil on the slide directly atop the center of the hole in the stage. Slowly move the nosepiece around until the oil immersion lens clicks into position. Be sure you rotate the nosepiece in the correct direction, viz. directly to the oil immersion lens and not to the high dry. If you do the latter, you will get oil on the high dry lens. Should this happen, inform the instructor and clean it immediately with lens paper and no harm will be done but do not allow oil to remain on this lens. Look through the eyepiece and focus on the object very carefully using fine focus only.
Figure 2. Trypanosoma lewisi from a rat. Protozoa141L.gif
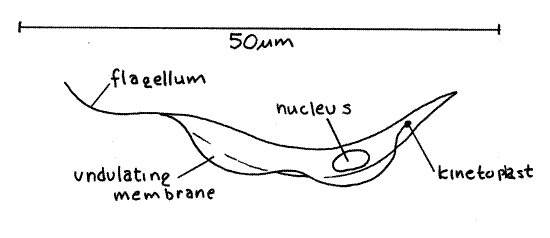
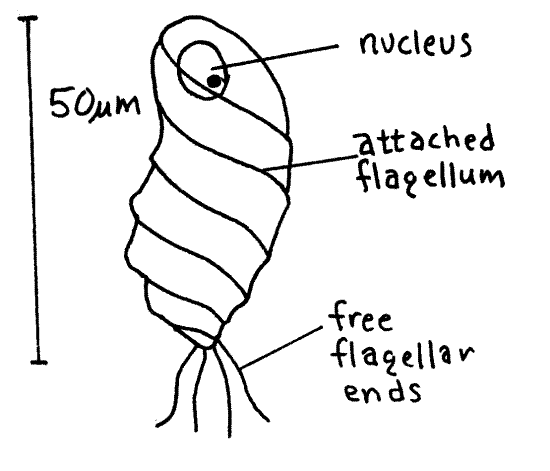
Note the elongate, narrow shape of the cell (Fig 2, 3-7C). The cells are flattened and you are probably looking at a wide side. You may occasionally see a cell that is turned on its edge to reveal its narrow margin. The cell is more or less comma-shaped, with convex and concave edges. The flagellum arises from the anterior end of the cell but is directed posteriorly so that it runs along the convex edge and trails from the posterior end. The flagellum is flattened and, together with the edge of the cell, forms a thin undulating membrane. The flagellum is attached to the cell membrane on the convex edge.
The flagellum arises from a basal body, often referred to as the kinetosome. The kinetoplast (= blepharoplast) is situated near the basal body (Fig 2, 3-7C). The kinetoplast and the basal body are too close together to be resolved with your microscope and are visible as a single dark spot at the anterior end of the cell. The kinetoplast consists of a region of high concentration of DNA at one end of an elongate mitochondrion. This mitochondrion is the only one in the cell but it is very long and extends the length of the cell (Fig 3-7C). Only the anterior region, with the heavy DNA concentration near the basal body, is visible (as the kinetoplast) in these preparations. The nucleus is located near the center of the cell.
When finished with the oil immersion lens, clean it carefully with lens paper. If you are using a prepared slide, clean it with a tissue.
References
Sleigh (1989), Vickerman et al (1991)
Trichonympha
Axostylata P, Parabasalea C, Hypermastigida O (Trichonympha, Holomastiogotes, Microspirotrichonympha )
Axostylata P, Oxymonadea C, (Pyrsonympha, Dinenympha )
Axostylates are complex gut symbionts, either parasites or commensals, occurring with a wide variety of vertebrate and invertebrate hosts. The characteristic skeletal or locomotory axostyle is a long, central bundle of microtubules (Fig 3-10A). As inhabitants of anaerobic environments (gut) they have no mitochondria and rely on glycolysis-based metabolic pathways rather than the Krebs cycle and electron transport chain. These are flagellates with four to thousands of flagella.
Several species are xylophagous, consuming wood, and inhabit the hindguts of some termites and roaches, where they are necessary for the chemical digestion of the cellulose in the insect's diet. Termites that harbor these commensals usually exhibit specific associations of several characteristic species. If suitable termites, such as Reticulitermes flaviceps in the eastern United States or Zootermopsis angusticollis in the western states, are available in the laboratory, the flagellates are easily removed and studied. Species of the hypermastigid genus, Trichonympha, are present in both of these termites and are good representatives of axostylate flagellates. Trichonympha agilis inhabits the common eastern termite, Reticulitermes, whereas Trichonympha campanula is found in Zootermitopsis. The exercise is written specifically for axostylates from Reticulitermes but can also be used in the West with Zootermitopsis.
3. Place a living termite, either Reticulitermes or Zootermitopsis , on a slide in a drop of 0.6% saline solution. While holding the thorax in place with a nadel or forceps, grasp the posterior tip of the abdomen with a fine forceps and pull it away from the remainder of the body. Most of the gut, including the hindgut, will come away with the forceps. Discard the remainder of the body, retaining the gut in the drop of saline. With minuten nadeln, tease the large, bulging hindgut apart to liberate the flagellates contained within. (Alternately, gut contents can be obtained by squeezing the abdomen gently with forceps until two drops of fluid appear at the tip of the abdomen but this is difficult and is usually less effective. The first drop probably will have no flagellates but the second should.)
Mix the gut contents with the saline and place a coverslip over the mixture. With the compound microscope, scan the slide for flagellates which should be readily visible if the light is adjusted properly. Find an area where protozoans are widely scattered and study some with high power. Several species of flagellates will be present on the slide.
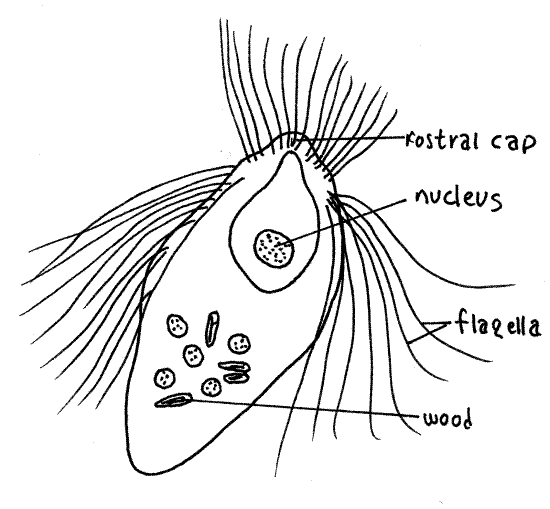
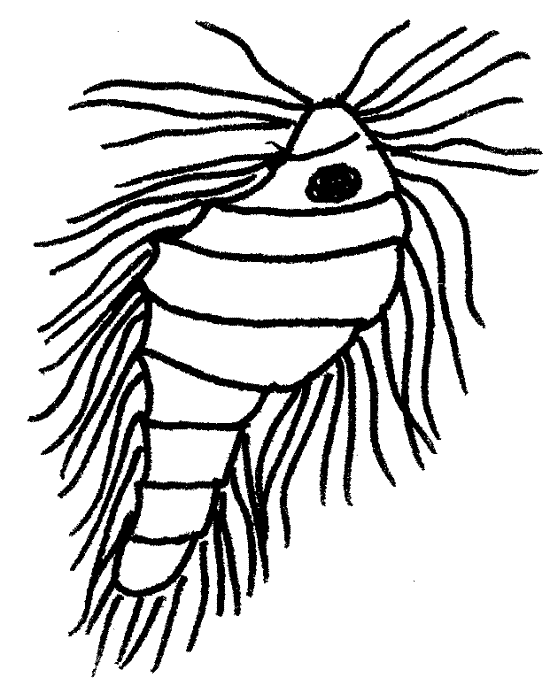
Look first for large hypermastigids in the genus Trichonympha. Trichonympha agilis (115 m m), from the eastern termite, is fusiform (Fig 3A). Trichonympha campanula , from the West, is pyriform with narrow anterior and bulging posterior ends (Fig 3-10B). The nucleus is central or anterior and is contained in a sac. In T. agilis the nucleus is very large but in T. campanula it is small. As is characteristic of all hypermastigids, abundant long flagella are found at the anterior end of the cell. Trichonympha is xylophagous and has particles of wood in vacuoles in the posterior end of the cell. The anterior end bears a rostral cap separated from the rest of the body by a shallow circumferential groove.
Figure 3A. The hypermastigid axostylate flagellate Trichonympha agilis. protozoa131L.gif
3b. Reticulitermes. There are several other flagellate species in your preparation and you may wish to identify them with the help of the figures. If your termite is Zootermitopsis, skip forward to 3c. Reticulitermes flaviceps has a fauna of five species including Trichonympha.
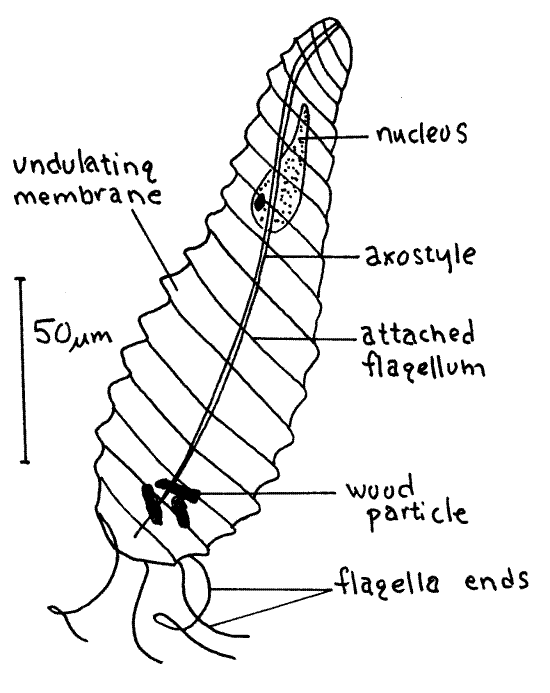
Pyrsonympha vertens (150 m m) is a common oxymonad species with a characteristic elongate pear or club shape (Fig 3B). It and Trichonympha are the largest species present in this termite. Pyrsonympha is narrow anteriorly with a swollen posterior end.
Four to eight flagella arise at the anterior end and spiral around the cell with their free ends trailing posteriorly. Pyrsonympha is easily distinguished from hypermastigids by its small number of flagella. The flagella are attached to the body along shallow grooves and, when beating, pull the body surface up to form undulating membranes. The undulations of these membranes are striking and conspicuous in living material but, when the cell dies they flatten and cannot be seen. The axostyle, a narrow central tube of microtubules, extends the length of the cell. It is difficult to see in living material but is conspicuous in stained specimens. The nucleus is large, anterior, and more or less pyriform. Pyrsonympha vertens is xylophagous and wood particles may be visible in the posterior end.
Figure 3B. The oxymonad axostylate Pyrosonympha vertens. protozoa140L.gif
Holomastigotes elongatum is a small (70 m m) hypermastigid (Fig 3C). It bears a few raised, helical bands of flagella that extend from the anterior to the posterior end. The nucleus is anterior. It is saprozoic rather than xylophagous and consequently does not contain wood particles.
Figure 3C. The hypermastigid axostylate Holomastigotes elongatum. protozoa130L.gif
Microspirotrichonympha porteri is a small (55 m m) pyriform hypermastigid with spiral rows of flagella at the anterior end (Fig 3D).
Figure 3D. The hypermastigid axostylate Microspirotrichonympha porteri. protozoa129L.gif
Dinenympha gracilis , an oxymonad, is a small species (50 m m) with an elongate body (Fig 3E). It is not xylophagous. Its four to eight flagella twist around the body and are free at the posterior end as are those of its relative, Pyrsonympha . It resembles Holomastigotes in shape and size but, being an oxymonad, has far fewer flagella.
Figure 3E. The oxymonad axostylate Dinenympha gracilis. protozoa139L.gif
3c. Zootermitopsis. (Omit if using Reticulitermes.) The western termite, Zootermopsis angusticollis, has a different axostylate fauna, including Trichonympha campanula (Fig 3-10B), which is similar to T. agilis. Tricercomitas termopsidis is a trichomonad characteristic of Zootermitopsis. It is very small (12 m m in length) with three anterior flagella and a long trailing flagellum. Hexamastix termopsidis is another small (11 m m) trichomonad. It has a conspicuous axostyle as well as five anterior and one trailing flagella. Streblomastix styrix (50 m m) is an oxymonad with four or so long flagella at the anterior end of a fusiform cell with spiral ridges.
3d. Add a small drop of acidified methyl green or iodine solution (Lugol’s or IKI) to the edge of the coverslip and draw it under the coverslip. Allow 5-10 minutes for the cells to absorb the stain and then study the cells affected by the stain. Look again for the structures mentioned above. Look for the axostyle in Pyrsonympha.
3e. The presence of wood in xylophagous species can be demonstrated by treating the flagellates with phloroglucinol and hydrochloric acid which causes the lignin in the wood to turn pink. Obtain termite hindgut contents by one of the methods described above and place them on a dry slide with a drop of phloroglucinol-HCl solution. Affix a coverslip and examine the slide for an area with a high concentration of large xylophagous axostylates such as Trichonympha or Pyrsonympha. Note the color of the cells. Let the slide sit for 30 minutes, adding phloroglucinol to the edge of the coverslip as it evaporates, then reexamine the large flagellates.
References
Cleveland (1924, 1926), Grell (1973), Honigberg (1970), Kirby (1937), Kudo (1966)
Peridinium
Alveolata P, Dinoflagellata sP
Alveolata includes three taxa, Dinoflagellata, Ciliophora, and Apicomplexa, characterized by alveoli in the pellicle and similarities in ribosomal DNA nucleotide sequences. Dinoflagellates (dino = terrible) are marine and freshwater, protozoans with two flagella. The 4000 Recent species may be either heterotrophic or autotrophic but the ancestor was a colorless heterotroph and pigmented forms arose through independent endosymbiotic associations with photosynthetic eukaryotes, probably at least three times. The nucleus is haploid and the chromosomes permanently attached to the nuclear envelope. Many dinoflagellates produce toxins and are responsible for a variety of harmful algal blooms including “red” tides of various colors and paralytic shellfish poisoning. Species with thick cellulose plates in the pellicle are said to be armored. Some species are bioluminescent.
4. If living material is available, prepare a wetmount using equal parts of culture and Protoslo (methyl cellulose) to slow the organisms and facilitate observation. If no living specimens are available, use a prepared slide of fixed and stained material. Examine the cells at 400X. The exercise is written specifically for armored dinoflagellates in the genus Peridinium but Ceratium and Glenodinium are similar and could also be used.
Peridinium, and other armored dinoflagellates, have an elaborate pellicle consisting of the cell membrane and intracellular cellulose armor plates (Fig 4, 3-11B). The plates are contained within large, flat alveoli just inside the cell membrane. The cellulose is intracellular and thus is not a cell wall. The sutures, or junctions between adjacent plates, may be visible. Look at the surface of the cell and try to discern the outlines of the armor plates. This is difficult in living, pigmented, moving cells but is easier in prepared slides.
Dinoflagellates have two flagella which arise from basal bodies close to each other on one side of the cell (Fig 1). One, the transverse flagellum, encircles the cell whereas the other, the longitudinal flagellum (= trailing flagellum), trails behind it.
Figure 4. The armored dinoflagellate, Peridinium. protozoa143L.gif
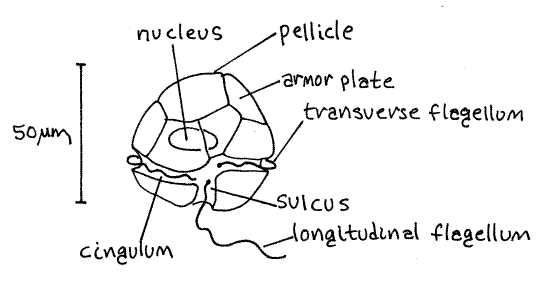
Each flagellum lies in a groove in the pellicle. One groove, the cingulum, or girdle, encircles the cell equatorially and contains the transverse flagellum. The sulcus is a short groove located on one side of the posterior half of the cell, perpendicular to the girdle. It contains the proximal portion of the trailing flagellum. If you are using prepared slides, you may need to look at several cells before you find one in which the sulcus is on the upper side where it can be seen. The transverse flagellum lies in the girdle and the trailing flagellum lies in the sulcus, from which it extends to trail behind the organism. The trailing flagellum is the easier of the two to see.
The single nucleus is located near the center of the cell but is difficult to see in living specimens. It is conspicuous in stained material. The photosynthetic pigments (chlorophyll a and c, and the xanthophyll carotenoid, peridinin) of dinoflagellates are in numerous small chloroplasts which, due to their color, are collectively visible in living specimens.
4a. The debris on the bottom of the culture jar may include empty pellicles which appear as colorless ghosts. Make a wetmount of a drop of this debris and look for these ghosts on which the armor plates, sutures, girdle, and sulcus are much easier to see. Prepared slides sometimes contain some of these ghosts.
References
Grell (1973), Sleigh (1989), Vickerman et al (1991)
Paramecium
Alveolata P, Ciliophora sP, Nassophorea C, Hymenostomata sC, Peniculida O, Parameciidae F
Ciliates are complex unicellular organisms with two types of nuclei and an elaborate pellicle and associated ciliature. Only axostylates rival ciliates in terms of cell complexity. They are heterotrophic although a few harbor photosynthetic endosymbionts (which have not become obligate chloroplasts, at least not yet). The 8000 known species exhibit great morphological heterogeneity and many are symbionts, either commensals or parasites. A well defined permanent mouth, or cytostome, occupies a fixed location where food vacuoles are formed. The locomotory apparatus consists of well coordinated cilia in longitudinal rows connected by an underlying infraciliature of basal bodies and fibers. The ciliature is divided into somatic ciliature covering the body and specialized oral ciliature in the mouth region. The degree to which the two types of ciliature are developed varies with taxon. The cilia are in rows known as kineties. The complex pellicle includes alveoli and is provided with trichocysts which can be discharged from the surface for defense or to assist in prey capture. Osmoregulation is via contractile vacuoles.
The study of Paramecium may be undertaken with living specimens or prepared slides. All Paramecium species are similar and any could be used for this exercise. The two most commonly used are Paramecium caudatum and P. multimicronucleatum both of which are large. Paramecium multimicronucleatum has several micronucleii whereas P. caudatum has only one. Paramecium multimicronucleatum also has several contractile vacuoles whereas P. caudatum has two. Paramecium bursaria is interesting, and might be chosen, because of its endosymbiotic unicellular green algae.
5. Paramecium is large, common, readily available, and easily maintained in the laboratory. These are active, slipper-shaped ciliates with a well developed feeding apparatus at the bottom of a deep invagination, the oral groove.
Prepare a wetmount by thoroughly mixing equal parts of Protoslo and Paramecium culture on the slide. Use as much Protoslo as possible without flooding the slide. Paramecium is very active and is difficult to immobilize. Ciliates are much faster than flagellates.
Observe the behavior of the organism at 100X and watch as it swims. Paramecium swims in a helix because its ciliary beat is oblique to the long axis of the body (Fig 3-17A). Paramecium can reverse the direction of its ciliary beat and move backwards. It can maneuver by locally regulating ciliary beat. Watch an individual and observe it making its characteristic avoidance reaction (Fig 3-17B). Upon encountering an obstacle or unpleasant patch of water, the organism backs away, then changes direction and swims off in the new direction. Describe the changes in ciliary beat the cell would have to make to accomplish this. Consider the locomotory apparatus and the movements of the organism.
Remove some of the water from the slide so the coverslip restricts the vertical motion of the cells and immobilizes them as much as possible. Find a relatively immobilized individual to study. Note its characteristic slipper shape (Fig 5, 3-19). The cytoplasm is held in this shape by the semi-rigid pellicle (Fig 3-14) consisting of the plasma membrane, trichocysts, alveoli, and infraciliature.
Note the difference in the shape of the two ends of the cell. The blunter end is anterior and the slightly pointed end is posterior. Paramecium swims with the anterior end forward.
Paramecium has well developed somatic ciliature consisting of cilia that cover the body surface in oblique rows, or kineties. With the light properly adjusted you can clearly see a halo of cilia surrounding the cell. Each cilium is a part of kinety, or row.
Find the large oral groove on one side of the middle of the cell. The groove is easy to see as the animal slowly rotates on its long axis. The entrance to the groove faces anteriorly and the cytostome (cyto = cell, stome = mouth) is at its posterior end. The groove is oriented obliquely across the cell and opens anteriorly.
Figure 5. The ciliate, Paramecium. The posterior contractile vacuole omitted for clarity. Protozoa137L.gif
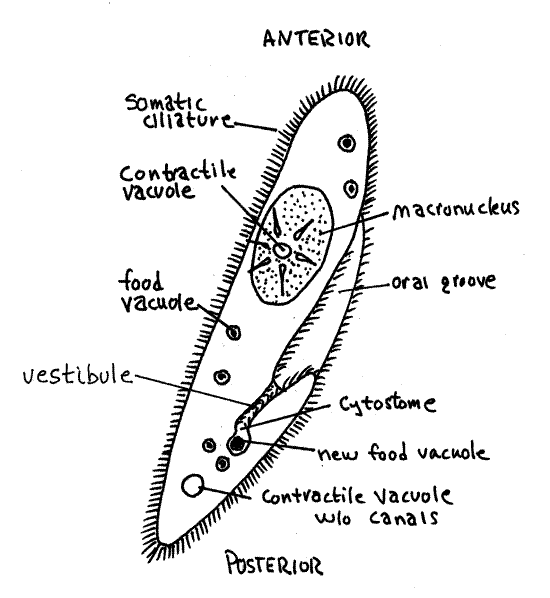
The cytostome opens into a fluid region of the cytoplasm known as the cytopharynx. The cilia of the oral groove move food particles (bacteria, small protozoa, organic particles) to the base of the groove where food vacuoles form in the cytopharynx. After formation, the vacuoles move in a circuitous route through the cell while the various events of digestion occur sequentially (Fig 3-21). The pH of the vacuole changes as it moves through the cell. It is initially highly acidic with a pH of 3 then the pH rises to about pH 5, at which digestion occurs. Eventually the vacuole arrives at the fixed cytoproct, or cytopyge, or cell anus, located just posterior to the cytostome (Fig 3-19). The cytoproct is visible only when digestive wastes are being exocytosed from it. Watch the cilia move particulate matter into the oral groove to form a food vacuole.
>5a. Prior to the laboratory period the teaching staff will add Congo red/yeast suspension to a subsample of the Paramecium culture. Make a wetmount of some Paramecium from this treated culture using Protoslo and a whisp of cotton. Find a relatively immobile specimen and observe it with 400X. Paramecium will ingest the red yeast particles. You may be able to see some of these particles enter the oral groove and become incorporated into food vacuoles but this may not happen as you watch. You will however see the food vacuoles formed earlier as they are rendered conspicuous by their color. Note carefully the color of the food vacuoles and see if there is more than one color of vacuole. Congo red is an indicator, red-orange at pH > 5 and blue-violet at pH < 3. <
Return to your original wetmount. The number of contractile vacuoles varies with species but there are at least two and they are large (Fig 5, 3-19). They occupy fixed positions with at least one at each end of the cell. Each consists of a large, central spherical vesicle from which several canals may radiate although canals are not always present. The vesicle fills and empties several times a minute, depending on the osmolarity of the medium. Watch one for a minute or so and observe it fill rapidly and then suddenly disappear as it empties. How long does it take to complete a cycle? What effect would increasing the osmolarity of the medium have on the length of a cycle? Design an experiment to test this hypothesis.
The single macronucleus is a large, irregular oval near the center of the cell. The micronucleus is small and located by the side of the macronucleus. The nuclei are difficult or impossible to see in living material as they are transparent and offer little contrast to the surrounding cytoplasm. The macronucleus is easy to see when stained, which you will soon do. You probably will not see the micronucleus.
The pellicle of Paramecium contains trichocysts which can be discharged in defense (Fig 3-15). A variety of chemical stimuli including weak acid, blue-black ink, and methylene blue may initiate discharge of the trichocysts in laboratory situations.
>5c. Place a drop of 0.5% aqueous methylene blue at the edge of the coverslip and draw it under the edge for a short distance. Watch the cells in the vicinity of the advancing methylene blue. Some of those that come in contact with it may fire their trichocysts. The methylene blue will also stain the macronucleus, making it easy to see. Careful focusing should reveal the parallel rows of infraciliature (kinities) and cilia.
>5d. If your Paramecium refused to discharge trichocysts, look at prepared slides of Paramecium with discharged trichocysts. <
>5e. Look at prepared slides of Paramecium undergoing asexual reproduction by cell division (fission). Note the orientation of the cleavage furrow with respect to the kineties. Fission is transverse, across kineties (Fig 3-22A). <
>5f. Observe prepared slides of Paramecium in conjugation (Fig 3-23). How can you quickly distinguish between Paramecium in fission and those in conjugation? <
Some Additional Ciliates
Stentor
Alveolata P, Ciliophora sP, Spirotrichea C, Heterotrichia sC, Stentoridae F
Stentor is a large contractile ciliate with well developed somatic (body) and oral (buccal) ciliature. The common blue stentor, Stentor coeruleus, often appears on floating docks, or vegetation in freshwater ponds, lakes, or reservoirs or it may be purchased from biological supply houses. It can be as large as 1-2 mm when fully extended.
Stentor lives attached to the substratum but can release its attachment and adopt a free-swimming existence. The body of a typical attached specimen is usually trumpet-shaped (Stentor was a mythological Greek herald who yelled louder than 50 ordinary men combined) or cylindrical (Fig 3-18F). An attached, trumpet-shaped Stentor can release its attachment, contract to about a third its original length, and swim. The shape change is due to contraction of protein fibers in the pellicle of the body. When swimming, Stentor is usually oval or pyriform.
Observe a stationary attached individual. Attachment is via the narrow basal end of the cell. The apical end flares out to form a large peristomial disc encircled by a peripheral ring of ciliary membranelles. At one side of the ring the membranelles spiral tightly downward to form a buccal cavity leading to the cytostome.
The macronucleus of most species, including S. coeruleus, resembles a string of beads. The micronucleus is not usually visible. The contractile vacuole is elongate. There is a well developed body, or somatic, ciliature.
The color of S. coeruleus is due to the pigment stentorin. Other species have other colors (rose, yellow-brown, amethyst-blue, other shades of blue) or are colorless and some have endosymbiotic zoochlorellae.
Add a drop of acidified methyl green to the edge of the coverslip and draw it under the coverslip to kill and stain the specimen. Allow a few minutes for the cells to absorb the stain and then look for the cellular structures, particularly the macronucleus, you could not find in the unstained material.
Euplotes
Alveolata P, Ciliophora sP, Spirotrichea C, Hypotrichia sC, Euplotidae F
Euplotes (Fig 3-18A) is a common, bacteriovorous, hypotrichous ciliate of fresh or salt water. Cilia are restricted to the ventral surface. Make a wetmount with a drop of culture and examine it with the compound microscope.
The ovoid body is flattened dorso-ventrally and is inflexible, maintaining a constant shape. The dorsal surface is convex. The flat ventral surface bears numerous conspicuous cirri, composed of fused cilia, whose movements resemble those of legs. Observe the use of the cirri in locomotion.
Also present on the ventral surface is a long band of oral membranelles, each short membranelle being a plate of fused cilia (polykinetids). The band like macronucleus is usually bent into a C-, or sometimes T-, shape.
Draw a drop of methylene blue under the coverslip, allow several minutes for the stain to be absorbed, and then study an individual looking for structures you could not see in the unstained, active specimens.
Didinium
Alveolata P, Ciliophora sP, Litostomatea C, Haptoria sC, Didiniidae F
Make a wetmount of a drop of water from the Didinium culture and examine it with the compound microscope.
The anterior end of these barrel-shaped ciliates (Fig 3-20A) culminates in a proboscis, surrounded by a ring of cilia, or pectinella. The expansible but temporary cytostome is located at the tip of the proboscis. Didinium has specialized extrusomes known as toxicysts in the proboscis. Toxicysts resemble trichocysts but have no barb aare equipped with toxin.
A second pectinella encircles the body near its equator. Oral cilia are absent and somatic ciliature is limited to the two pectinellae. The macronucleus is horseshoe shaped. Two to four micronuclei are present and a large contractile vacuole is present at the posterior end of the body.
Draw a drop of methylene blue under the coverslip, allow several minutes for the stain to be absorbed, and then study a Didinium again looking for structures you could not see in the unstained, swimming specimens.
Didinium is a carnivore preying exclusively on Paramecium. Didinium does not actively hunt its prey instead collides with it accidentally. Upon colliding, Didinium establishes and maintains contact using short rods, known as pexicysts, in the proboscis. Toxicysts are fired into the prey, initiating a localized death of cytoplasm and discharge of prey trichocysts. The prey is gradually drawn into the predator’s cytostome.
On a fresh slide mix a small drop of Paramecium culture with a drop of Didinium culture. Observe the combined drop using high power of the dissecting microscope. Look for the events described above and describe what you observe. Watch for the discharge of trichocysts by Paramecium.
Vorticella
Alveolata P, Ciliophora sP, Oligohymenophorea C, Peritricha sC, Vorticellidae F
Peritrichs are ciliates with well-developed buccal ciliature and reduced body ciliature (Fig 3-18C,D). Adult peritrichs are attached to the substratum via a stalk, which may be contractile. Some may swim free in the water and be mistaken for rotifers. Some are solitary and some are colonial, with many individuals sharing a branching stalk attached to the substratum. Members of Vorticellidae have a contractile stalk whereas Epistylidae has non-contractile stalks and Ophrydiidae has no stalks at all. Vorticella is a solitary species (Fig 3-18B) and Carchesium is colonial (Fig 3-18E). Vorticella or a relative can usually be found attached to vegetation, rocks, docks, or other firm substrata in fresh water. Cultures are available from biological supply companies.
Make a wetmount and examine Vorticella or Carchesium with 400X of the compound microscope. Note the stalk which attaches the bell-shaped cell body to the substratum. The stalk is an extracellular secretion of the basal region of the cell body known as the scopula. It contains an array of longitudinal elastic fibrils in its periphery. Its core is the spasmoneme, a longitudinal bundle of contractile fibers. These fibers are made of the contractile protein spasmin rather than acto-myosin and apparently do not use ATP as an energy source. The spasmoneme is variously described as being contained in an evagination of the cell body that extends the length of the stalk or as being extracellular. Individual spasmin fibers, separate from each other to extend longitudinally below the pellicle over the surface of the body. Contraction of the spasmin fibers results in the rapid coiling of the stalk and retraction of the cell body. Recovery and reextension is much slower.
Observe the cell body of an extended Vorticella. The large, distal end of the bell is a disc known as the peristome. The peristome supports an elaborate oral ciliature consisting of two peripheral rings of peristomial cilia and a ciliary membranelle. Currents created by peristomial cilia move food, chiefly bacteria, into the buccal cavity opening on one side of the disc. The buccal cavity is an invagination leading to the cytostome where food vacuoles form. Somatic cilia are absent.
Tap on the stage of the microscope to induce contraction of the spasmoneme. Note the rapidity of the contraction and the helical shape of the stalk and spasmoneme after contraction. Compare the length of the contracted stalk with that of the fully extended stalk. Note that the oral cilia are not evident in contracted individuals. They are folded under a peripheral shelf, or collar, of cytoplasm.
The macronucleus is an elongate rod bent more or less into a “C”. The small micronucleus is difficult to discern. A large contractile vacuole should be visible in the cytoplasm between the buccal cavity and macronucleus. The vacuole discharges periodically into the buccal cavity. The cytoplasm usually contains numerous small food vacuoles. Look for the parallel spasmin fibers on the sides of the cell body and observe that they converge in the scopula to form the spasmoneme of the stalk. Note that the body, other than the peristome, has no cilia.
Add a drop of acidified methyl green to the edge of the coverslip and draw it into the preparation. Be aware that this will kill everything on the slide. Allow a few minutes for the cells to absorb the stain and then look for the cellular structures you could not find in the unstained material.
Bursaria
Alveolata P, Ciliophora sP, Colpodea C, Bursariidae F
Bursaria is a very large, ovoid or reniform (kidney-shaped) ciliate reaching 1000 μ m in length. Prepare a wetmount using sand grains to support the coverslip.
Bursaria has a complete and uniformly distributed somatic ciliature with spiraling kineties. The peristome is a deep, funnel-like invagination extending from the anterior end beyond the middle of the body into the posterior end. The macronucleus is a long curved rod. Between 10-34 micronuclei are present. Numerous contractile vacuoles are distributed along the sides and posterior border.
Draw a drop of methylene blue under the coverslip, allow time for organelles to absorb the stain and then examine the slide looking for structures, such as the macronucleus, that you may not have seen prior to staining.
Bursaria is a raptor feeding on other protozoa including ciliates such as Paramecium and Spirostomum.
On a fresh slide mix a small drop of Paramecium culture with a drop of Bursaria culture. Observe the combined drop using high power of the dissecting microscope. Look for evidence predation of Paramecium by Bursaria. Describe the process and compare feeding by Bursaria with that by Didinium.
References
Bullough (1958), Burbanck (1950), Corliss (1979), Freeman and Bracegirdle (1971), Grell (1973), Ho (1978), Kudo 1966), Lynn and Corliss (1991)
Monocystis lumbrici
Alveolata P, Apicomplexa sP, Gregarinea C, Eugregarinida O, Acephalina sO, Monocystidae F
The 5000 known species of Apicomplexa (= sporozoa) are animal parasites and many cause economically or medically important diseases. Apicomplexans have an apical complex constructed of microtubules which is visible only with the electron microscope (Fig 3-24). The apical complex, for which the taxon is obviously named, attaches the parasite to the host cell. Only one nucleus is present and cilia and flagella are absent except in male gametes. In general, apicomplexans are intra- or extracellular parasites with complex life cycles including sexual and asexual phases. Both vertebrates and invertebrates may be parasitized. Malaria, which is considered by the World Health Organization to be the world's most important human parasitic disease, is caused by Plasmodium, a large genus of Apicomplexa.
Because of its considerable public health importance, Plasmodium (Hemarozoea C)is often studied in introductory invertebrate zoology laboratories. Plasmodium, however, usually must be studied using prepared slides. As an alternative, the gregarine, Monocystis is used in this manual as the exemplar of Apicomplexa. Monocystis is a common parasite of earthworms and can be studied alive. It inhabits the seminal vesicles of mature oligochaete worms where it feeds on newly formed spermatozoa. Gregarines are mostly extracellular parasites of the body cavities of invertebrates or lower vertebrates. There is usually only one host in the life cycle which nevertheless includes sexual and asexual phases.
6. Monocystis lumbrici (= M. agilis) is a large (200 m m) apicomplexan in the taxon Gregarinea C parasitizing the seminal vesicles of the nightcrawler, Lumbricus terrestris (Fig 3-25). Monocystis can usually be found in earthworms reared and sold for fishing bait. Dissected earthworms or their seminal vesicles may be provided by your instructor or you may be asked to do the dissection yourself. If the latter is the case, select the largest worm available as the incidence of infection is positively correlated with size. Anesthetize the worm by placing it in a dish of 5% ethanol.
Familiarize yourself with the basic anatomy of the host worm, referring to the earthworm exercise (Annelida chapter) in this manual for assistance as needed. You must be able to distinguish dorsal from ventral and anterior from posterior. Find the clitellum, which is a conspicuous wide band of thickened epithelium near the anterior end of a mature worm. Examine the clitellum and note that it is curved on three sides but one surface is flattened. The flattened surface is ventral. Further, the dorsal surface of the worm is darker than the ventral. Note that the earthworm is segmented. The segments are referred to by number with the anteriormost segment being the first.
Use your finest scissors to open the anterior end of the worm with a shallow, mid-dorsal, longitudinal incision extending from the clitellum to the tip of the head. Internal pressure may force one or more of yellowish seminal vesicles out of the incision. If this happens, you need dissect no further and may remove one of the vesicles with a pair of fine scissors. Place the vesicle on a slide and cover it with 0.6% saline. With experience it is possible to remove the seminal vesicles rapidly by making a short dorsal incision in the vicinity of segments 9-15 only.
If the vesicles do not emerge through the incision, place the worm in a small dissecting pan and pin the body wall to the wax with #1 insect pins. Insert the pins into the wax at 45 ° angles so you have plenty of working room in the body cavity. The three pairs of seminal vesicles are large, globular, pale yellow organs lying on either side of the gut in the anterior end of the body in the vicinity of segments 9-15. With the exception of the gut itself, the seminal vesicles are the largest structures in the body cavity but they are not all the same size. Use fine forceps or scissors to remove a large piece of a seminal vesicle. Place the vesicle on a microscope slide and flood it with a few drops of 0.6% saline solution.
With fine needles and forceps, tease the seminal vesicle apart, swish the tissue around in the saline, and then discard the tissue, retaining the cloudy fluid on the slide. Add a few drops of 0.5% methylene blue or 1% neutral red to the suspension remaining on the slide and mix. Use a fine pipet to remove most of the liquid from the slide and then add a coverslip to that which remains. Study the preparation with the compound microscope.
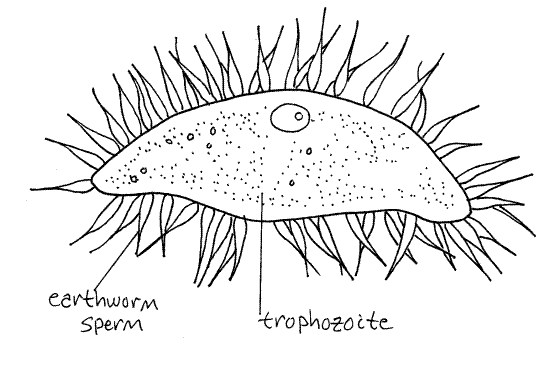
The most common objects on the slide should be the sperm balls of the earthworm. These are large flagellated, lobed spheres. The flagellated lobes are clusters of sperm around a central nurse cell. If your worm is heavily infected, many of the objects you see will be the various stages in the Monocystis life cycle. If your worm is only lightly infected you will have to make a systematic search of the slide looking for each life history stage, although you may not find all stages on a single slide. If necessary, make wet mounts from the other seminal vesicles of your worm, sacrifice another worm, exchange slides with other students, or look at examples found by other students. Try to find each of the stages in the life cycle (except individual sporozoites).
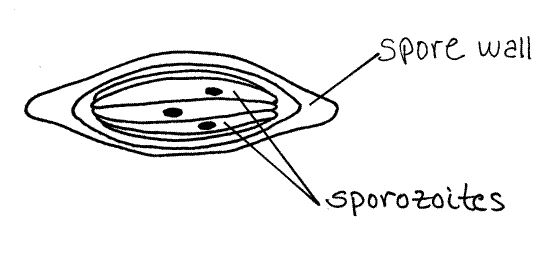
Earthworms are infected by Monocystis when they ingest its spores in the soil (Fig 3-25). The small, spindle shaped, thick-walled (20 m m) spores (Fig 6A) contain sporozoites (Fig 6B) which emerge from the spore case upon reaching the worm’s gizzard. The sporozoites move posteriorly with the gut contents into the anterior stomach-intestine which they exit by penetrating the gut wall. They enter the dorsal blood vessel and are carried anteriorly to the esophageal region. Here they exit the hemal system, enter a seminal vesicle, and penetrate the sperm-forming cells in the wall of the vesicle.
Figure 6A. Monocystis life cycle stages. Spore. Protozoa262L.gif
The sporozoites feed on the developing spermatocytes in the wall of the seminal vesicle. Later the move into the lumen of the vesicle where they mature into the vegetative cells, or trophozoites. If your worm is infected with Monocystis, many, or most, of the sperm balls will contain a young trophozoite in its interior (Fig 6C). The young trophozoites feed on the developing sperms and their nurse cell and grow larger, eventually destroying the sperm ball. The trophozoite is the feeding stage of the life cycle, hence the name (troph = food). Young trophozoites are covered by the remains of degenerating sperm cells and appear bristly with flagella but the flagella belong to the sperm, not the parasite.
Figure 6B. Monocystis life cycle stages. Sporozoite. Protozoa263L.gif
After consuming the sperm ball, the young trophozoite becomes a mature trophozoite (Fig 6D). In the relatively simple Monocystis life cycle the trophozoites do not undergo schizogony to produce additional trophozoites as do the trophozoites of many other apicomplexans.
Figure 6C. Monocystis life cycle stages. A young trophozoite in a sperm ball. The flagella belong to the sperm, not the trophozoite. Protozoa264L.gif
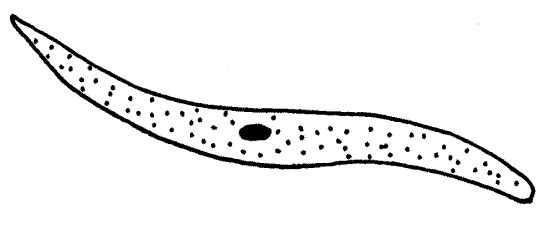
Mature trophozoites are large (200 m m), fusiform unicells and, if present, may be the most conspicuous objects on your slide. The exterior is smooth, with no spermatozoa or flagella on the surface. Mature trophozoites are mobile but they die quickly on the slide. Their movements are reminiscent of euglenoid motion but the mechanism by which it is accomplished is not understood. Trophozoites have a large nucleus with a large nucleolus. The interior endoplasm is granular but the thin outer layer of ectoplasm is hyaline. The nucleus is easier to see if you remove some fluid from the preparation so the coverslip flattens the trophozoite slightly. Trophozoites are easily recognized and, since this stage is relatively long lived, are usually common and easy to find.
Figure 6D. Monocystis life cycle stages. A mature trophozoite. The trophozoites will next undergo syzygy. Protozoa265L.gif
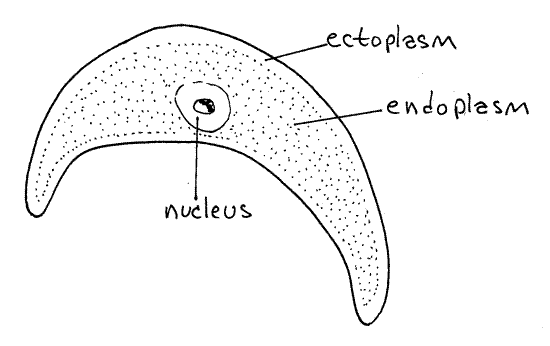
Figure 6E. Monocystis life cycle stages. A gametocyst formed by the union of two trophozoites of opposite mating type. The gamonts will undergo gamogony to produce gametes. Protozoa266L.gif
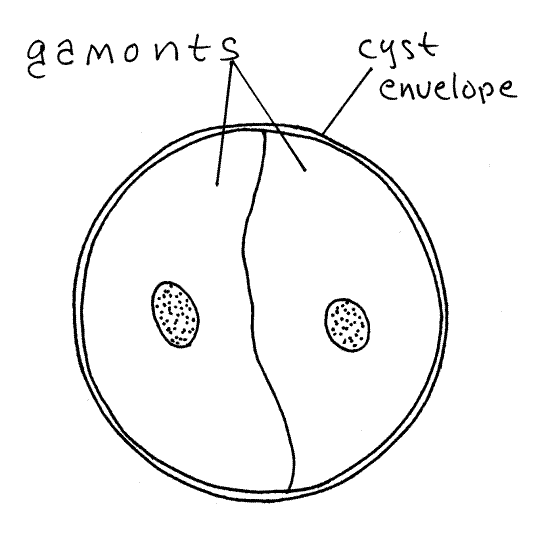
Two (sometimes more) mature trophozoites join in a non-sexual union known as syzygy and together form a spherical gametocyst enclosed in a single shared cyst envelope (Fig 6E). The two cells remain independent and do not unite. The former trophozoites are now called gamonts (or gametocytes) and they are of opposite mating types, for convenience referred to as male and female although they are not visibly different and do not have different reproductive roles. Adjust the light so you can see the contents of the cysts. Find a cyst in which gamonts can be seen (Fig 6E). If your preparation is fresh, you will likely see the gamonts slowly rotating in the cyst but you may not be able to distinguish one from the other. Formation of gamonts and secretion of the cyst occur rapidly. The stage is a brief one and as a consequence it is scarce and hard to find. Gametocysts are always spheres but, depending on maturity, enclose a variety of life cycle stages. Various stages of gametocysts are described below and should be evident in your preparation.
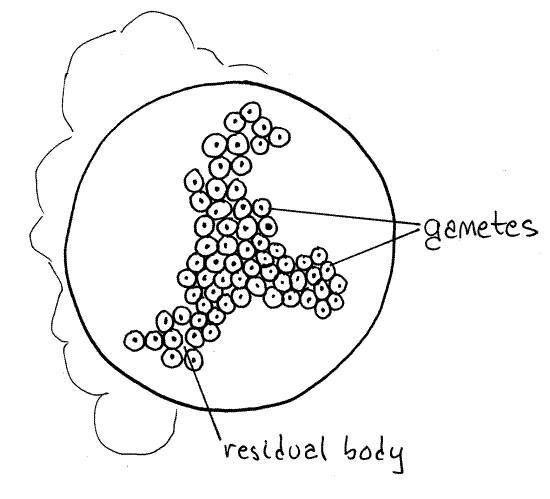
In a budding process called gamogony, each of the two gamonts undergoes multiple nuclear divisions to produce many nuclei. Each nucleus moves to the periphery of its gamont and separates from it, taking a little cytoplasm with it, and becomes a gamete. Look for a gametocyst with small nuclei budding away from the periphery of the much larger gamont. The remaining, unused cytoplasm of the gamont is the residual body. The gametes produced by the two gamonts are of both mating types, either male or female, corresponding with the mating type of parent gamont.
Figure 6F. Monocystis life cycle stages. A gametocyst immediately following gamogony and filled with gametes of two mating types. Next, gametes of opposite mating type will unite to form zygotes. Protozoa267L.gif
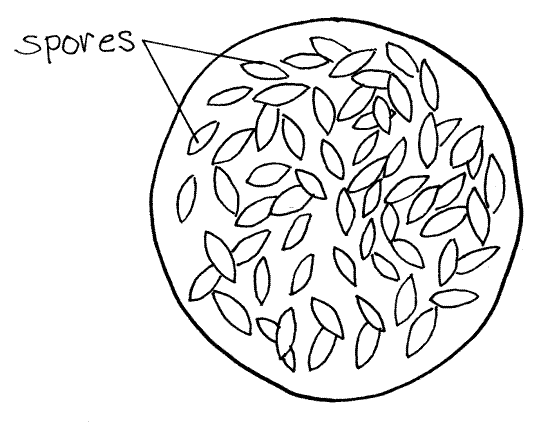
While still within the cyst envelope, gametes of opposite mating types (sexes) unite in a sexual union called syngamy (syn=together, gamy=gamete) to form a diploid zygote (Fig 6G). The zygote is the only diploid phase of the life cycle. Within each gametocyst many pairs of gametes unite so that many zygotes are formed. Each zygote secretes a thick extracellular spore wall (Fig 6A) around itself to form the previously mentioned spore. Your preparation may have several large, spherical gametocysts filled with hundreds of spindle-shaped spores (Fig 6H). This is a persistent stage and many examples should be present.
Within the spore, the diploid zygote undergoes a combination of meiotic and mitotic divisions to produce eight haploid daughter cells by a process known as sporogony. Each of the eight daughters differentiates into a sporozoite but remains enclosed in the spore wall with its seven sisters (Fig 6A). The hundreds of spores in each gametocyst are released at about this time. You may also see isolated spores scattered about in the preparation (Fig 6A). (These are spores produced within the worm, not those ingested by the worm.) The gametocyst, or spores if they have been released, exit the earthworm through the vas deferens and wait in the soil until they are ingested by another worm. In the new host the sporozoites emerge from the spore, enter the hemal system and infect the seminal vesicles. You will not see individual sporozoites in your preparation.
Figure 6G. Monocystis life cycle stages. A gametocyst with zygotes. Protozoa268L.gif
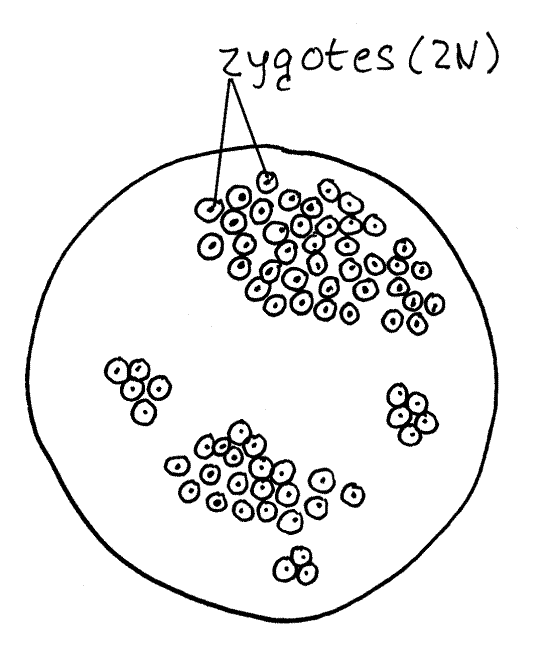
Figure 6H. Monocystis life cycle stages. A late stage gametocyst containing spores. Protozoa269L.gif
References
Bullough (1958), Burbanck (1950), Cable (1977), Lankester (1903), Schmidt and Roberts (1989)
Opalina
Stramenopila, Opalinata P, Opalinida O, Opalinidae F
The opalines are large symbionts with an opalescent pellicle and many parallel rows of short flagella. They are gut commensals of anuran amphibians and fishes and have at least two, but often hundreds, of identical nuclei.
7. Examine a commercially prepared slide or, if you wish to study living material, pith a grass frog ( Rana pipiens ) or other anuran, open the abdomen, remove the rectum (expanded posterior end of the gut), and place it in a small dish of 0.7% saline. Open the rectum and use a pipet to remove a drop or two of its contents. Place the drops on a slide, add 2 drops of Protoslo, mix, and affix a coverslip. Scan the preparation for Opalina. In general, the incidence of opalinid infection in grass frogs is less than 10% so it may be necessary to sacrifice several animals. The rectal contents of a single infected frog can be used by several students and the wetmounts, once prepared, can also be shared. Sometimes recently sacrificed frogs are available from the physiology lab.
Figure 7. Opalina. Protozoa128L.gif
Opalina is a very large (500 m m or more in length), flat, variously shaped but more or less oval, flagellated cell. It is unmistakable and you will have no trouble recognizing it. Note the short flagella in oblique spiral rows and the numerous small nuclei (Fig 7). The pellicle is folded into oblique ridges and grooves from which the flagella arise. In living specimens look for the opalescence for which the group is named. The cytoplasm is divided into an outer clear ectoplasm and an inner granular endoplasm. The endoplasm contains the numerous nuclei and numerous smaller, darkly stained granules. To living material, add acidified methyl green to stain the nuclei. You may find some individuals undergoing binary fission.
References
Hyman (1940), Metcalf (1923), Sleigh (1989), Wessenberg (1961)
Amoeba
Ameboid Protozoa, Amebas, Amoebozoa, Lobosea, naked amebas, Amoebida O, Amoebidae F
The “ameboid” protozoans, characterized by common possession of pseudopods and formerly assigned to the taxon Sarcodina, are no longer considered to be a monophyletic group. Unfortunately there is not yet a comprehensive phylogeny, with taxon names, to explain the evolutionary relationships among these taxa.
Ameboid protozoans include marine, terrestrial, and freshwater unicellular heterotrophic protozoans with pseudopodia. Their cells are the simplest among the protozoa with one to many nuclei and many have internal or external skeletons. The higher taxa included in “ameboid protozoa” are amebas, Foraminiferea, and Actinopoda.
Ameboid cytoplasm is divided into a thin layer of stiff, hyaline ectoplasm covering the remaining, internal, fluid, granular endoplasm. Pseudopods may be thick lobopodia comprising endoplasm and ectoplasm, or slender filopodia, lacking endoplasm. Although amebas have no internal skeleton, a secreted, external, organic test, to which debris or secreted silicon may be added, is present in testate species. Naked amebas have no test. Contractile vacuoles are present in freshwater species but lacking in marine amebas.
Amebas occur in most aquatic or moist habitats including soil and in symbiosis with other organisms. Some species, such as Entamoeba histolytica, the causative agent of amoebic dysentery, are human pathogens.
8. Amoeba proteus is a large and easily obtained naked ameba. It is best studied using living specimens but commercially prepared slides can be used if necessary. Remove a drop of debris from the bottom of the Amoeba culture jar and place it on a slide. Scatter a few (5-10 is sufficient!) grains of fine beach sand in the drop and add a coverslip. The sand protects the amebas from being crushed by the coverslip. Close the iris diaphragm partly to improve contrast and make a systematic search for Amoeba (Fig 8, 3-28A). Be sure you are focused on the surface of the slide and that you do not have too much light. On your first attempt you may confuse inanimate debris with amebas. The debris in commercial cultures is usually brown whereas amebae are gray but both appear to be shapeless. If close observation reveals very slow movement, you probably have an Amoeba. Anything moving rapidly is not Amoeba.
With the light carefully adjusted, use 400X to observe the organism. The granular, gray cytoplasm is enclosed in a simple cell membrane. In Amoeba the pellicle is simply the unadorned membrane with no infrastructure.
The cytoplasm consists of a thin, hyaline ectoplasm forming a stiff, outer layer immediately inside the cell membrane. Lobes of cytoplasm, known as pseudopodia, extend from the organism. The pseudopodia of Amoeba are thick blunt lobopodia that may appear at any location on the surface of the cell. The ectoplasm at the tip of each pseudopodium is a little thicker than it is elsewhere and forms a hyaline cap. The remainder of the cytoplasm is the more fluid, granular endoplasm. Most of the cytoplasm is endoplasm. Observe the formation of pseudopodia on the forward, or anterior, end of the cell.
Look for the nucleus near the center of the cell. It is large and shaped like a thick disk or biscuit, although the shape varies somewhat.
Figure 8. Amoeba proteus. Protozoa136L.gif
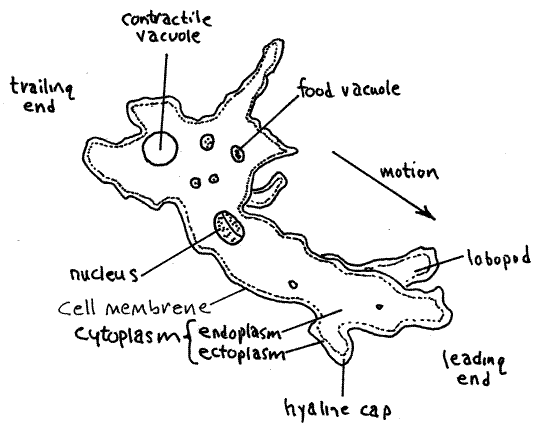
A large, hyaline, spherical contractile vacuole is located in the trailing end of the cell. The size of the vacuole changes as it fills rapidly with water from the cytoplasm and then empties suddenly to the outside. Obviously it won’t be visible immediately after it has emptied. The vacuole is also difficult to observe if it happens to be on the side of the cell away from you. Both situations change, however, and an empty vacuole will quickly fill to become visible and a hidden vacuole will eventually move around to your side of the cell where you can see it. Watch a vacuole as it fills with water and then suddenly disappears when it empties to the exterior. You might want to design an experiment to test the effect of environmental osmolarity on the rate of vacuole evacuation.
The endoplasm contains numerous, mostly spherical food vacuoles of various sizes. These are formed by phagocytosis of the small unicells that are the food of Amoeba. Within the vacuoles food items are in various stages of digestion and those recently phagocytized may still be recognizable. The food vacuoles are darker than the contractile vacuole and are usually opaque.
There are numerous other inclusions in the endoplasm giving it its characteristic granular appearance. Among these are inorganic crystals and oil droplets.
References
Brooks (1989), Pearse et al (1987), Sherman and Sherman (1976), Sleigh (1989), Wessenberg (1961)
Arcella
Ameboid Protozoa, Amebas, Amoebozoa, Lobosea C, testate amebas, Arcellinida O,Arcellidae F
The shelled, or testate, amebas are protected by an external organic shell which they secrete and sometimes augment with additional siliceous secretions or debris. Arcella vulgaris (vulgar = common) is a shelled freshwater ameba that inhabits vegetation and the bottom of quiet fresh water. It is best studied using living material but prepared slides may be substituted if necessary.
9. Make a wetmount of material from the bottom of the Arcella culture jar using sand grains to support the coverslip. Search the slide for Arcella (Fig 9, 3-29A,B). Most of the specimens will be seen from above (polar view) but you should make a point of examining debris for cells you can see from the side. Both views are useful.
Figure 9. Arcella vulgaris. A, Polar view . B, Side view . Protozoa135L.gif
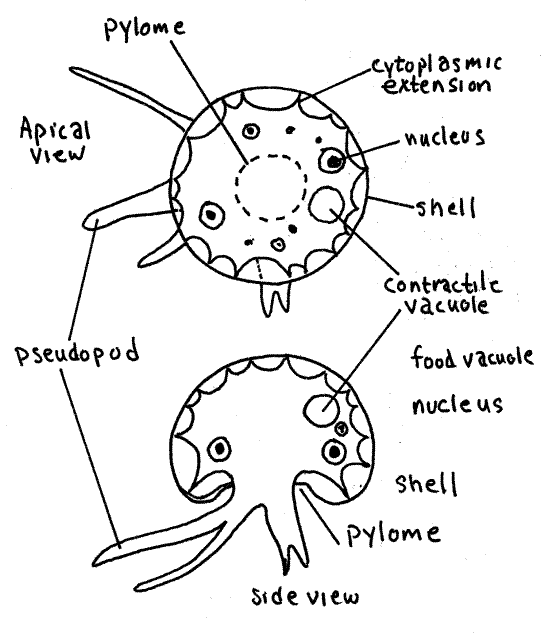
Arcella secretes and inhabits a transparent, brownish, proteinaceous, extracellular test. The hemispherical test has an opening, the pylome, in the center of the lower surface. The cell occupies most of the space inside the test but there is a sizable gap between the cell and the test. The cell is attached to the inside of the test by threadlike cytoplasmic extensions.
Arcella has slender, sometimes branched pseudopodia which it extends through the pylome. The pseudopods are transparent, colorless lobopodia. They are not always evident but you should search until you find a specimen in which they are visible. Adjust the light carefully and look for pseudopods around the periphery of the shell. The pseudopodia extend, retract, and change shape quickly; quick for an ameba anyway.
Contractile vacuoles eliminate the water that enters continuously from the hyposmotic environment. There may be several small contractile vacuoles or a single large one and they are easy to see when close to the surface. They are hyaline and spherical. You can be sure you have found a contractile vacuole if it suddenly disappears while you are watching it. A momentary disturbance of the neighboring cytoplasm is associated with the emptying of the vacuole. If at first you cannot see the vacuole, watch for the sudden, brief movement of cytoplasm and then watch for the reappearance of the vacuole in the vicinity of the disturbance.
Arcella vulgaris has two nuclei which are usually on opposite sides of the cell. These are best seen on prepared slides of fixed and stained material or you can stain your preparation with acidified methyl green.
If some of your specimens have been accidentally turned upside down, you may notice the rapid appearance of small, black, spherical gas bubbles in the cytoplasm. These are produced as a righting response to help the organism turn itself back over.
References
Burbanck (1950), Grell (1973), Page (1982)
Difflugia
Ameboid Protozoa, Amebas, Amoebozoa, Lobosea C, testate amebas, Arcellinida O, Difflugiidae F
Difflugia is another testate amoeba, one that differs from Arcella in the composition of its test (Fig 3-29D). Make a wetmount of debris from the bottom of the Difflugia culture and observe it with the compound microscope. Use a few sand grains to support the coverslip and protect the tests from being crushed.
Difflugia constructs and inhabits a test composed of sand grains, diatom frustules, or other particles cemented together. The test is usually ovoid or pyriform and has an opening, the pylome, at the smaller end.
The body fills most of the space within the test and is attached to it by long cytoplasmic strands. Organelles are usually difficult to observe because of the opaque test and/or zoochlorellae in the cytoplasm (more later). There is one large nucleus. Contractile and food vacuoles are present in the cytoplasm.
Some species feed on the long, helical, strap-like chloroplasts of the filamentous green alga, Spirogyra. Commercially available cultures of Difflugia are sometimes shipped with Spirogyra filaments. Try to observe feeding if your culture has Spirogyra.
The pseudopods are cylindrical lobopodia of which as many as six may be seen extending from the pylome. The lobopods may be branched or not. Difflugia undergoes an “Inch-worm” like locomotion using pseudopodia extended from the pylome. The body lies on its side with the side of the test resting against the substratum and the pylome facing in the direction of motion. A pseudopod is extended forward and attached to the substratum. The body and test are then pulled forward toward the apical, attached tip of the pseudopod. Another pseudopod is then extended in a similar fashion and the body pulled to its apical attachment point. Observe and describe Difflugia locomotion.
Like Arcella, Difflugia can produce a gas bubble and with it lift itself above the substratum. Most species inhabit the bottom of quiet fresh waters. Some are found in soil. Some lacustrine (lake dwellers) Difflugia species are meroplanktonic (part-time inhabitant of the plankton) and spend the winter and spring encysted on the bottom and the summer and early fall in the plankton.
Difflugia often harbors endosymbiotic zoochlorellae in its cytoplasm. In such instances the cytoplasm may be bright green. The symbionts are cells of the green unicellular alga Chlorella.
Burbanck (1950), Grell (1973), Kudo (1966),
Globigerina
Amoeboid protozoa, Foraminiferea P, Rotaliina C
Foraminifereans, or “forams”, are amoeboid protozoans found primarily in marine habitats where they may be either benthic or planktonic. Forams construct and inhabit a test of calcium carbonate, organic material, or debris. The surface of the shell is often sculptured with numerous small pits, pores, spines, bumps, teeth, or little peaks. The tests of most species are composed of many chambers, called locules, and are said to be multilocular. The locules are of increasing size and are added as the organism grows, so that the oldest locule is the smallest. The single cell occupies all the locules.
The shell has a large aperture from which cytoplasm protrudes. The exterior of the shell is invested with a thin covering of cytoplasm. The exterior cytoplasm gives rise to numerous very long, very narrow, often branching and anastomosing filiform reticulopodia (reticulo=net). In multilocular forams, including Globigerina, the test is perforated by tiny pores through which cytoplasm extends (Fig 3-30B, 3-35-A,B). Reticulopods are the characteristic pseudopodia of Foraminiferea.
10. Living forams are not usually available for classroom studies and prepared slides of the empty tests of calcareous species are used instead. Such slides may be mixtures, known as “strews” (strew = to spread thin by scattering), of many species from marine sediments, or they may contain a single species. Globigerina is a common planktonic genus and is well represented on most commercially prepared slides (Fig 10, 3-30B). The multiple locules (chambers) of its calcareous shell are spheres of increasing size arranged in a spiral.
Find one or more representatives of Globigerina on your slide. Note the spiral progression of spherical locules. The largest locule is the youngest and bears the aperture, which is visible in properly oriented specimens. Carefully use high power to focus on the surface of a locule and note the surface sculpture, if any. Globigerina may have minute, sharp peaks with pits at their apices.
Figure 10. The planktonic marine foram Globigerina. Protozoa138L.gif
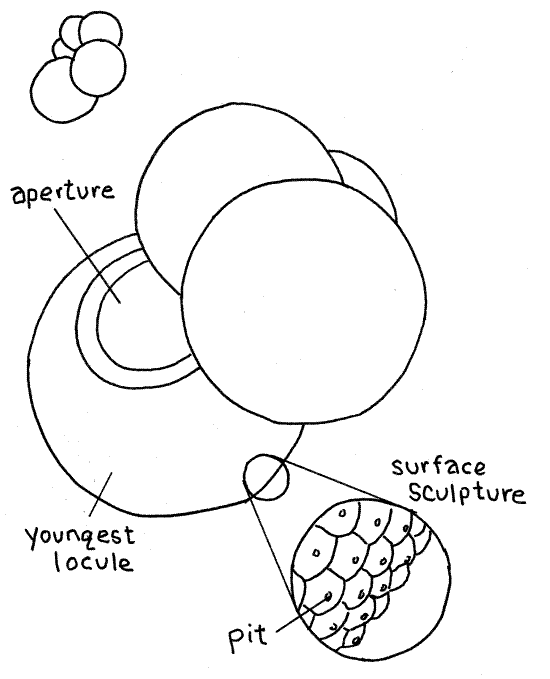
Look at the shells of other species of forams if any are present on your slide. Note the many shapes and arrangements of locules. Look for features, such as the aperture, pits, surface sculpture, locules, and secondary apertures.
References
Cushman (1948), Hyman (1940), Kudo (1966), Moore (1964)
Radiolaria
Ameboid Protozoa, Actinopoda P, Polycystinea C,
Actinopoda P includes taxa of spherical, marine and freshwater, heterotrophic protozoa whose pseudopodia include characteristic axopodia with a stiffness due to a core of microtubules.
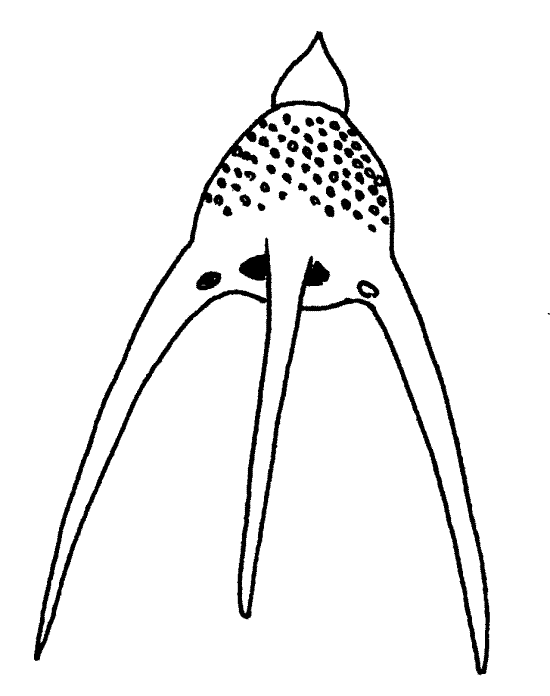
Three actinopod taxa, Polycistinea, Acantharea, and Phaeodarea, are collectively known as “ radiolarians”. Polycistinea includes most of the familiar radiolarians. All radiolarians are marine and have axopodia radiating from the cell (Fig 3-31A). An organic test and mineral skeleton are both present. Most are planktonic and most are spherical although the skeleton often is not.
Although axopodia are characteristic of radiolarians, other types of pseudopodia are also present and contribute importantly to the unusual morphology of these organisms. The cytoplasm is divided into an outer cortex and an inner medulla by the secreted, extracellular, organic test (capsule). The test is the boundary between medulla and cortex (Fig 3-35C). The cortex outside the test is actually a dense mass of vacuolated filopodia that arise in the medulla and penetrate the test. The cortex is often vacuolated and frequently contains photosynthetic zooxanthellae. The nucleus (or nuclei) is in the central medulla. The microtubular axes of the axopodia originate in the medulla, pass through pores in the test and skeleton and then extend, covered with a thin layer of cytoplasm, from the surface of the cell.
Radiolarians have an intracellular mineral, usually siliceous, skeleton in addition to the extracellular test (Fig 3-31B, C). Paradoxically, the skeleton is in the cortex, which is outside the test but the skeleton is nevertheless intracellular because it is inside the cytoplasm of the filopodia that make up the cortex (refer to Fig 3-35C for help in understanding the complex and potentially confusing morphology of radiolarians). Spines of the skeleton, if any, also pass through the capsule and extend, covered with cytoplasm, from the surface of the cortex. This skeleton is a conspicuous and characteristic feature of radiolarians and persists after death, frequently accumulating on marine bottoms as radiolarian ooze.
Figure 11A. Siliceous skeleton of Trochodiscus . Protozoa133L.gif
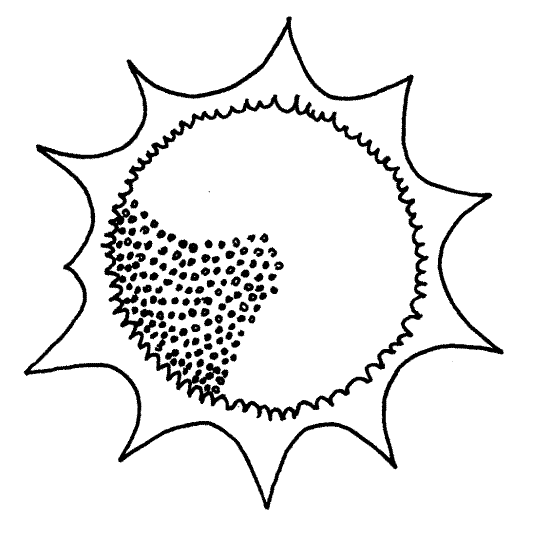
11. The traditional laboratory study of radiolarians, like that of forams, consists of examination of slide preparations of empty skeletons. These preparations, known as “radiolarian strews”, contain only the mineral skeletons of the organisms. Examine such a slide and try to find some complete skeletons to study (Fig 11). Although they are beautiful and instructive, they provide no insight into the bizarre morphology of intact radiolarians (Fig 3-35C).
Note the diversity on the slide. Many genera are represented. Observe the conspicuous pores in the skeletons through which the microtubular cores of the axopodia pass.
Since you are seeing only the skeletons of these organisms, you should mentally reconstruct the rather complicated spatial relationships between the skeleton, test, medulla, cortex, and axopodia.
Figure 11B. Siliceous skeleton of Podocyrtis. Protozoa134L.gif
References
Borradaile et al (1961), Cachon and Cachon (1982), Grell (1973), Hyman (1940), Margulis et al (1990), Moore (1954)
Actinosphaerium
Actinopoda P, Heliozoea C, Actinophryida O, Actinosphaeridae F
Actinopoda includes spherical, marine and freshwater, heterotrophic protozoa whose pseudopodia include characteristic axopodia with a stiffness due to a core of microtubules.
Heliozoans, or sun animalcules, are freshwater, marine, and terrestrial (in moist habitats such as mosses) protists which, like radiolarians, have radiating axopodia supported by microtubular cores (Fig 3-33, 3-34, 3-35D). Heliozoans further resemble the radiolarians in the subdivision of the cytoplasm into inner medulla and outer vacuolated cortex but there is no organic test separating the two. The cortex is composed, as is that of radiolarians, of a dense layer of filopodia. Sometimes an extracellular skeleton is present enclosing the outer border of the cortex (Fig 3-34A,B, 3-35D). Such heliozoans are said to be testate. The microtubular cores of the axopodia arise in the medulla and the nucleus or nuclei are medullar. Endosymbiotic zoochlorellae may also be present in the medulla.
12. Heliozoans are best studied using living specimens but prepared slides will suffice if this is not practical. The two most readily available genera are Actinosphaerium and Actinophrys. Actinophrys is smaller than Actinosphaerium, has relatively longer axopodia, and its cytoplasm is less vacuolated.
Prepare a wet mount of Actinosphaerium (or Actinophrys) from the debris in the culture jar using a few (!) grains of fine beach sand to support the coverslip. Actinosphaerium is an atestate heliozoan lacking the extracelular skeleton. Find a specimen and examine it with high power (Fig 12). The cell is spherical. The outer cortex is slightly paler than the inner medulla and is composed of filopodia with abundant fluid-filled vacuoles which give it a frothy appearance. These particular vacuoles are not contractile, rather are thought to reduce the specific gravity of the cell making it easier to remain suspended in freshwater.
Actinosphaerium has several hundred small nuclei in the medulla but they are difficult to see. Actinophrys has one large nucleus, located in the center of the medulla.
Numerous thin, conspicuous, needlelike axopodia radiate from the surface of the sphere. Each axopodium is composed of a core of microtubules, the axial rod, which arises in the medulla, and a thin covering of cytoplasm enclosed in the cell membrane. Focus carefully with properly adjusted light and look for the axial rod, which may be difficult to see, in the core of an axopodium. Patient observation will show that the cytoplasm is moving slowly in opposite directions on opposite sides of the axopodium. Pick a spot on one of the axopodia and watch it for a minute or so to see if it moves. Each axopodium apparently arises in association with a nucleus deep in the endoplasm. The length of the axopodia is changeable and, in fact, these organisms can move using a rolling locomotion accomplished by shortening the axopodia on the leading margin. Axopodia are shortened by depolymerizing the microtubules and lengthened by polymerization.
Figure 12. The atestate heliozoan, Actinosphaerium. Protozoa132L.gif
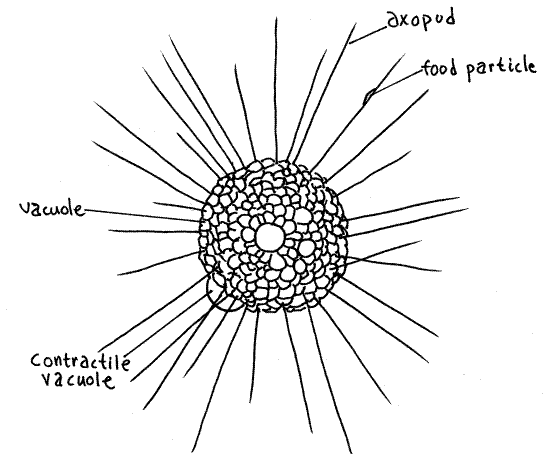
Large contractile vacuoles occupy positions on the periphery of the cell but may not be visible if they are on the hemisphere opposite you. These vacuoles are large, transparent sacs bulging away from the surface of the ectoplasm. If you find one, watch it fill and empty through a few cycles.
Actinosphaerium feeds on a variety of small algae, diatoms, protozoa, and small metazoans such as rotifers. Upon blundering into one of the axopodia, the prey sticks to the pseudopod and becomes immobile, apparently paralyzed. The axopodium bends or shortens so the food can be phagocytized. The prey, and the resulting food vacuoles, may be quite large.
12a. There are probably some small flagellates or other potential food items in the culture with Actinosphaerium and you may be able to observe their capture by the axopodia. Watch and describe the process.
12b. Under acidic conditions the microtubules depolymerize. They will collapse if treated with 1% acetic acid. After you have finished your other observations draw a little 1% acetic acid under the coverslip and observe the response of the axopodia.
*Hyphenated call-outs, such as this one, refer to figures in Ruppert, Fox, and Barnes (2004). Those without hyphenation refer to figures embedded in this exercise.
References
Balamuth W. 1950. in Brown FA, Selected invertebrate types. Wiley, New York. 597p.
Borradaile LA, Potts FA, Eastham LES, Saunders JT. 1959. The Invertebrata, A manual for use of students, 3 rd ed. Cambridge University Press, Cambridge. 795 pp.
Brooks WK. 1890. Handbook of Invertebrate Zoology. Bradlee Whidden, Boston. 352 pp.
Brown FA (ed). 1950. Selected Invertebrate Types. Wiley, New York. 597p.
Buetow DE . 1968a. The Biology of Euglena, volume I, General Biology and Ultrastructure. Academic Press, New York. 361p.
Buetow DE . 1968b. The Biology of Euglena, volume II, Biochemistry. Academic Press, New York. 417p.
Bullough WS . 1958. Practical invertebrate anatomy, 2 nd ed. MacMillan, London. 483 pp.
Burbanck WD. 1950. in Brown FA . Selected Invertebrate Types. Wiley, New York. 597p.
Cable RM . 1977. An illustrated laboratory manual of parasitology, 5 th ed. Burgess, Minneapolis. 275 pp.
Cachon J, Cachon M . 1982. Actinopoda, Pp 553-568 in Parker SP, Synopsis and classification of living organisms, vol 1. McGraw-Hill, New York. 1166 pp.
Cleveland LR. 1924. The physiological and symbiotic relationships between the intestinal protozoa of termites and their host, with special reference to Reticulitermes flaviceps Kollar. Biol. Bull. 46:203-227.
Cleveland LR. 1926. Symbiosis among animals with special reference to termites and their intestinal flagellates. Quart. Rev. Biol. 1:51-60.
Corliss JO. 1979. The Ciliates Protozoa: Characterization, Classification, and Guide to the Literature, 2 nd ed. Pergamon, New York.
Cushman JA. 1948. Foraminifera. Harvard, Cambridge. 605p.
Freeman WH, Bracegirdle B. 1971. An atlas of invertebrate structure. Heinemann, London. 129 pp.
Grell KG. 1973. Protozoology. Springer-Verlag. New York. 554 pp.
Harrison FW, Corliss JO (eds.). 1991. Microscopic Anatomy of Invertebrates volume 1, Protozoa. Wiley-Liss, New York. 493p.
Ho J-S . 1978. Laboratory manual for invertebrate zoology emphasizing marine forms. Hwong Publ, Los Alamitos Ca. 152 pp.
Honigberg BM. 1970. Protozoa associated with termites and their role in digestion. In K. Krishna & F. Weesner (eds). Biology of Termites, vol. 2. Academic Press. New York. pp 1-36.
Hyman LH. 1940. The Invertebrates: Protozoa through Ctenophora, vol. I. McGraw Hill, New York. 726 p.
Kirby H. 1937. Host-parasite relations in the distribution of Protozoa in termites. Univ. California Publ. Zool., 41:189-212.
Kudo RR. 1966. Protozoology 5 th ed. Thomas. Springfield. 1174 pp.
Lankester R.(ed.) 1909. A Treatise on Zoology Part I. Introduction and Protozoa (1) . Black, London. 296p.
Leedale GF. 1967. Euglenoid Flagellates. Prentice-Hall, Englewood Cliffs, 242p.
Lynn DH, Corliss JO. 1991. Ciliophora, pp. 333-468 in Harrison FW, Corliss JO (eds.). Microscopic Anatomy of Invertebrates volume 1, Protozoa. Wiley-Liss, New York. 493p.
Manwell RD. 1961. Introduction to Protozoology. St. Martin’s Press, New York. 642p.
Margulis L. et al . 1990. Handbook of Protoctista. Jones, Bartlett, Boston. 1024 pp.
Metcalf MM . 1923. The opalinid ciliate infusoria. Bull. U.S. Nat. Mus. 120:1-484.
Moore RC (ed). 1954 . Treatise on Invertebrate Paleontology, Part D. Protista 3, Protozoa (Chiefly Radiolaria and Tintinnia). Geological Society of America, Boulder. 195p.
Moore RC (ed). 1964. Treatise on Invertebrate Paleontology, part C, Protista 2 Sarcodina, Chiefly “Thecamoebians” and Foramininerida, vol. 2. Geological Society of America, Boulder. Pp. C511-C900.
Page FC. 1982. Lobosa, Pp. 510-517 in Parker SP, Synopsis and classification of living organisms, vol 1. McGraw-Hill, New York. 1166 pp.
Pearse VI, Pearse J, Buchsbaum M, Buchsbaum R. 1987. Living invertebrates. Blackwell (Boxwell), Palo Alto. 848 pp.
Pringsheim EG. 1956. Contributions towards a monograph of the genus Euglena. Nova Acta Leopoldina 18:1-168.
Schmidt GD, Roberts LS. 1989. Fundamentals of Parasitology. Times Mirror Mosby, St. Louis. 750p.
Sherman IW, Sherman VG. 1976. The Invertebrates: Form and Function, A Laboratory Guide (2 nd ed). MacMillan, New York.
Sleigh M. 1989. Protozoa and Other Protists. Routledge, Chapman, Hall, New York. 320p.
Ruppert EE, Fox RS, Barnes RB. 2004. Invertebrate Zoology, A functional evolutionary approach, 7 th ed. Brooks Cole Thomson, Belmont CA. 963 pp.
Vickerman K. 1982. Zoomastigophora, in Parker, S., Synopsis and Classification of Living Organisms, vol 1. McGraw-Hill. New York. 1166 p.
Vickerman K, Brugerolle G, Mignot J-P. 1991. Mastigophora, pp. 13-160 in Harrison FW, Corliss JO (eds). Microscopic anatomy of invertebrates, vol. 1, Protozoa. Wiley-Liss, New York. 493 pp.
Wessenberg H. 1961. Studies on the life cycle and morphogenesis of Opalina. Univ. California Publ. Zool. 61:315-370.
Supplies
compound microscope
clean slides and coverslips
Pasteur pipets
fine beach sand
Congo red-yeast suspension
absorbent cotton
5 % non-denatured ethanol
iridectomy scissors
small dissecting pan
# 1 stainless steel insect pins
Dropper Bottles with: Protoslo, IKI (iodine potassium iodide), 0.6 % saline, phloroglucinol-HCL, 0.5% aqueous methylene blue, acidified methyl green, 1 % acetic acid
Cultures: Euglena gracilis or E. viridis, Peridinium or Glenodinium, Paramecium caudatum, P. multimicronucleatum, or P. bursaria, Euplotes, Vorticella, Bursaria, Stentor, Didinium, Amoeba, Arcella vulgaris, Difflugia, Actinosphaerium or Actinophrys
Prepared slides: Trypanosoma, Paramecium discharged trichocysts, Paramecium fission, Paramecium conjugation, foraminiferan strew and/or Globigerina, radiolarian strew
Other Living Specimens: Termites, either Reticulitermes (in the East) or Zootermitopsis (in the West), earthworms (for Monocystis exercise), grass frogs (for Opalina exercise)