Invertebrate Anatomy OnLine
Plumatella repens ©
Fresh-water Bryozoan
28may2007
Copyright 2004 by
Richard Fox
Lander University
Preface
This is one of many exercises available from Invertebrate Anatomy OnLine , an Internet laboratory manual for courses in Invertebrate Zoology. Additional exercises, a glossary, and chapters on supplies and laboratory techniques are also available at this site. Terminology and phylogeny used in these exercises correspond to usage in the Invertebrate Zoology textbook by Ruppert, Fox, and Barnes (2004). Hyphenated figure callouts refer to figures in the textbook. Callouts that are not hyphenated refer to figures embedded in the exercise. The glossary includes terms from this textbook as well as the laboratory exercises.
Systematics
Lophophorata SP, Bryozoa P, Phylactolaemata C, Plumatellida O, Plumatellidae F (Fig 25-18B, 9-26)
Lophophorata SP
Lophophorata includes three taxa, Phoronida, Bryozoa, and Brachiopoda, sharing several morphological characteristics. Some zoologists include Kamptozoa in this group. The first three taxa possess a funnel-shaped anterior ring of ciliated tentacles known as a lophophore (Fig 25-2, 25-25A). The lophophore surrounds the mouth and is an upstream collecting system for suspension feeding. Its tentacles are hollow with extensions of a coelomic space thought to be the mesocoel. The gut is U-shaped with the anterior mouth at the center of the lophophore. The anus is also anterior, but is dorsal to the mouth, outside the ring of the lophophore (Fig 25-2A).
The lophophoral tentacles bear two types of cilia. Frontal cilia on the inside face of the tentacles extend into the interior of the lophophore, whereas lateral cilia on the sides of the tentacles extend into the gap between adjacent tentacles. The mouth is at the bottom (apex) of the lophophore funnel and is encircled by the ring of tentacles. The feeding current is generated by the lateral cilia. In lophophorates and many filter feeding deuterostomes (but not Kamptozoa) water enters the open end of the lophophore, moves toward the mouth, and then exits laterally between the tentacles (Fig 25-25A). Food particles are captured on the upstream side of the tentacles and transported to the mouth by the frontal cilia. In cross section the lophophore may be horseshoe-shaped or circular (Fig 25-4).
Lophophorates inhabit a secreted enclosure, tube, shell, or zooecium that may be organic or mineral. The body is divided into two parts, the mesosome and metasome, each with a coelomic space. The small mesosome is the region of the lophophore and the much larger metasome is the trunk and accounts for most of the body. The tiny anterior epistome is sometimes considered to be a third body region homologous to the protosome of early deuterostomes. Lophophorates are suspension feeders and most are marine but some occur in freshwater.
Bryozoa P
Bryozoa (moss animals) are derived, almost always colonial, lophophorates. Colonies are composed of individuals, or zooids, which are usually less than 0.5 mm in length. Each zooid inhabits a secreted box, the zooecium, into which is can retract. Because of their small size, hemal, excretory, and respiratory systems are absent.
Colonies are usually attached to firm substrata and may be encrusting layers a single zooid thick, or foliose and leaflike, or fruticose (bushlike) and branching (Fig 25-15). Some, such asZoobotryon, are stoloniferous and consist of tangles of long slender stolons (which are zooids). Bryozoans often resemble seaweeds (or mosses) with which they are frequently confused by the public. Alone among lophophorates, Bryozoa includes freshwater representatives.
The lophophore may be circular or horseshoe shaped in cross section. A small epistome may be present anterior to the mouth. The zooecium can be composed of chitin, calcium carbonate, or gelatinous organic compounds. Many bryozoans are polymorphic with zooids specialized for different functions.
Phylactolaemata C
Phylactolaemate bryozoans form globular or stolonate colonies in a variety of exclusively fresh water habitats. About 20 species are known from North America. The cylindrical zooids are circular in cross section and the lophophore is (almost always) horseshoe-shaped (That of Fredericella is circular) and bears a single row of hollow tentacles. The arms of the horseshoe extend dorsally and an intertentacular membrane connects the bases of the tentacles. A small epistome overhangs the mouth, which is ventral to it. The epistome is thought by some to be a protosome. The body wall musculature consists of layers of both circular and longitudinal fibers. The zooecium (= ectocyst) is organic, sclerotized or flexible, sometimes gelatinous, but never calcified. The trunk coelom is continuous, sometimes via communication pores through septa, with that of adjacent zooids although septa are absent in many phylactolaemates. Colonies are monomorphic with no specialized zooids. Phylactolaemates are hermaphroditic (as are most gymnolaemates). Statoblasts, which are clonally produced overwintering bodies, are characteristic of phylactolaemates.
Laboratory specimens
Plumatella repens is a common, cosmopolitan, stoloniferous species but is only one of several members of the genus. It is the most common freshwater bryozoan in North America.Plumatella repens, or a congener, can often be collected from firm substrates in low light intensity habitats on docks, woody debris, or rocks in lakes and reservoirs. Some are found in streams, especially those with low current velocity. Other freshwater bryozoans can also be used for this exercise. Most freshwater bryozoan colonies reach their peak of development in the summer and die with the approach of winter although in milder climates some (Paludicella, Lophopus, and Fredericella) may persist through the winter.
Plumatella and Fredericella are common stoloniferous or encrusting genera. Pectinatella (Pectinella gelatinosa is now Asajirella gelatinosa) is the most common of the genera forming gelatinous globular colonies. Cristatella is also gelatinous but forms smaller colonies than Pectinatella. Cristatella is of special interest because its colonies are motile and can crawl slowly over the substratum.
If living specimens cannot be secured locally. prepared slides are available from biological supply houses. As usual, living specimens should be used if at all possible, if for no other reason than for the opportunity to appreciate the exquisite beauty of an extended lophophore. Even if living material is available it may be useful to supplement its study with occasional reference to prepared slides.
Anatomy
Bryozoans live in colonies, or zoaria, composed of numerous, sometimes thousands, of individuals known as zooids. Use the dissecting microscope on low power to examine a piece of a healthy colony in a 8-cm culture dish of lake water. The specimen should be completely immersed in the water. If living material is not available, study a commercially prepared slide with 40X of the compound microscope or dissecting microscope.
Observe the overall structure of the zoarium (colony) (Fig 1). It is composed of numerous cylindrical zooids arising from a recumbent stolon which, in life, is attached to the substratum. The zooids extend into the water above the substratum.
Zooids
The monomorphic colony is composed entirely of unspecialized autozooids (Fig 1, 25-18B) typical of bryozoans and equipped with retractile lophophore, gut, funiculus, and reproductive equipment. Find and study several zooids that can be viewed from the side.
A specialized terminology has developed to facilitate discussion of the major bryozoan body regions. Each zooid consists of the outer immobile cystid (or body wall) and the inner extensible polypide consisting of the major organ systems. The cystid is the outermost, secreted, nonliving, cuticular ectocyst plus the underlying endocyst consisting of the epidermis, basal lamina, muscles, and mesothelium. The ectocyst is often referred to as the zooecium. The polypide consists chiefly of the lophophore, digestive system, nervous system, and associated musculature. The polypide and cystid are joined to each other by a mesothelial funicular system, the tentacle sheath, and the lophophore retractor muscles.
Stolons and new zooids arise by clonal budding on the ventral margin of existing zooids resulting in the growth of the colony over the substratum. In this regard, phylactolaemates differ from gymnolaemates in which budding and growth occur on the dorsal surface of the zooid.
Each zooid, and the region of stolon from which it arises, may be partly separated from adjacent regions by incomplete septa although in P. repens septa are usually not present.
The epidermis of a bryozoan zooid secretes a nonliving, extracellular cuticle, or zooecium (zoo = animal, oikos = house, thus “animal house”), outside itself. In phylactolaemate bryozoans the zooecium (which is also known as te ectocyst) may be gelatinous, as in Pectinatella, or membranous, as in Plumatella. Find the tubular, transparent, flexible zooeciumenclosing the zooids and stolon. The zooecium is continuous over the colony.
Figure 1. A zooid and portion of the stolon of Plumatella viewed from the left. The left limb of the lophophore has been removed to reveal the right limb in greater clarity. Drawn from fixed, mounted material. Bryozoa88L.gif
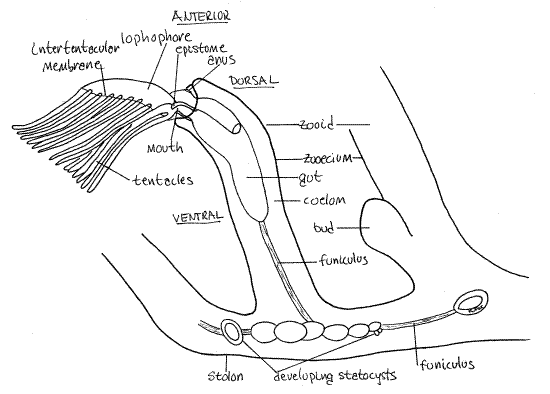
The most conspicuous features of the zooid are its zooecium, lophophore, and gut. The zooecium is underlain by the epidermis which secreted it, a layer of circular muscle, a layer of longitudinal muscle, and the mesothelium. Together the zooecium, epidermis, muscles, and mesothelium constitute the body wall. The body wall is known as the cystid to distinguish it from the rest of the zooid, which is called the polypide.
The polypide is the part of the zooid not contributing to the body wall and consists chiefly of the lophophore (an array of ciliated tentacles), the gut, gonads, and funiculus. Most of the polypide is movable and can be extended or retracted whereas the cystid in immovable. The spacious interior of the zooid and stolon is the trunk coelom, or metacoel. It is continuous throughout the colony, although in some species it may be incompletely partitioned by partial septa penetrated by communicating pores (Fig 25-23C). Septa will probably not be visible in your specimens.
Lophophore
Find some zooids with tentacles extended and look at one from the side (Fig 1). The free end of the zooid is anterior and the attached end is posterior. Whereas the stolon consists almost entirely of cystid and coelom (metacoel), most of the zooid is polypide (and coelom), which nearly fills the interior of the zooecium (Fig 1, 25-17).
The distal, or free and unattached, end of the zooecium bears a circular opening, the orifice, through which the lophophore can be extended and retracted The lophophoral tentacles are attached to the distal end of the zooid and are borne on a circular or horseshoe shaped ridge of tissue known as the lophophore. (The term lophophore originally referred only to this ridge but is now usually used to mean both the ridge and its tentacles.) The lophophore is the mesosome of the lophophorate body and the remainder of the zooid is the metasome. Like the metasome, the mesosome contains a coelomic space, in this case the mesocoel, or lophophoral coelom (Fig 127-17). Diverticula of the mesocoel extend into the lophophoral tentacles. The bases of the tentacles are joined by a low intertentacular membrane (Fig 1).
The lophophore, along with the remainder of the anterior end of the polypide form an introvert that can be extended and retracted. Many of the zooids in your colony will have retracted lophophores but some will usually be extended making the tentacles easy to see. A pair of lophophore retractor muscles can quickly pull the introvert back into the zooecium. Feeding requires that the lophophore be extended. Extension is accomplished by contracting the circular muscles in the body wall. This pressurizes the coelomic fluid to pop the introvert out of the orifice.
>1a. If your specimen is living, use the microneedle to touch gently the tentacles of an extended zooid and watch the response produced by the retractor muscles. Continue watching patiently until the lophophore is reextended. <
Find an autozooid with the lophophore extended. In almost all phylactolaemates the lophophore tentacles form horseshoe-shaped feeding structure at the apex of the introvert (Fig 2). The tentacles are hollow with extensions of the mesocoel and are ciliated. The cilia generate the feeding current. The mouth lies at the center of the horseshoe of tentacles.
>1b. If you have living specimens in a culture dish, place a drop of a carmine suspension in the water so there is a cloud of small red particles in the vicinity of a group of extended zooids. With low power watch the movement of particles with respect to the lophophore. From which region do particles enter the horseshoe? Do all particles entering the horseshoe end up in the gut? Can particles be ejected after entering the lophophore? What are some mechanisms for rejection of particles from the horseshoe?
Observe the activity of the cilia on the tentacles. What can you tell of the distribution of these cilia? The inner surfaces of the tentacles bear frontal cilia which are responsible for moving captured food particles toward the mouth. The sides of the tentacles bear lateral cilia. The lateral cilia generate the feeding current. Describe the direction of the feeding current. Are cilia present on the outside edges of the tentacles? <
Bryozoans are noted for the characteristic flicking motion of their tentacles. Observe this motion in one of your animals. It is accomplished by the longitudinal muscles in the body wall of the tentacles.
Find a zooid oriented so you can look straight down into the lophophore and locate the mouth. From this view you can see clearly the horseshoe shape of the lophophore (Fig 2). See if you can see the lateral cilia from this viewpoint.
Figure 2. Anterior view of the horseshoe-shaped lophophore of Pectinatella. Bryozoa90L.gif
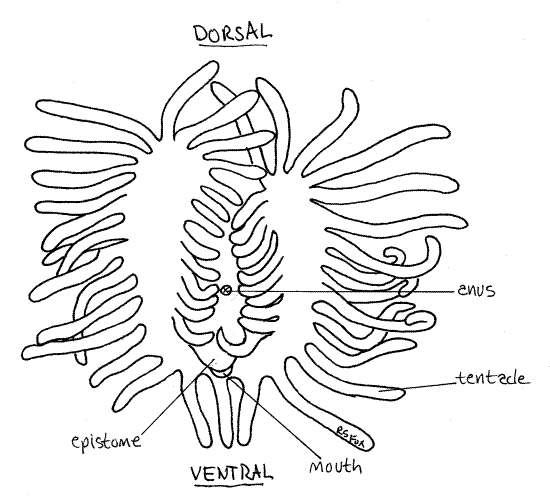
Digestive System
Look at a zooid from the side (Fig 1, 3, 25-18). Most of the tissue in a zooid is the lophophore and the digestive system. The gut is U-shaped with both mouth and anus located anteriorly, in the vicinity of the lophophore. Such an arrangement is common in tube-dwelling animals as it prevents feces from accumulating in the blind-ending posterior end of the tube.
The gut consists of pharynx, stomach, and rectum separated by sphincters. The anterior mouth is encircled by the tentacles of the lophophore. The mouth opens into a relatively shortpharynx with a thick wall of tall columnar epithelial cells. The initial, anterior region of the pharynx, into which the mouth opens, is ciliated and is sometimes referred to as the buccal cavity. Food particles accumulate in the pharynx before being admitted to the stomach.
The pharynx opens into the large stomach (Fig 3) and the two are separated by a sphincter, the cardiac valve. The stomach occupies the bend in the U-shaped gut and consists of three regions. The cylindrical cardia is the first stomach region and the pharynx opens into it. The cardia in turn opens into the large posterior cecum. This region occupies the turn in the “ U” of the gut and it is here that the gut reverses direction. Most absorption occurs in the cecum and the funiculus attaches to it. In phylactolaemates the third region, the pylorus, is short or absent. InPlumatella it is a very short tube connecting the cecum with the rectum. A sphincter, the pyloric valve, separates the pylorus from the rectum. The narrow, tubular rectum ascends to the anus(Fig 1, 3, 25-18B). Feces are stored in the rectum and formed into fecal pellets. The anus is at the base of the lophophore on the dorsal side of the animal. It is outside the ring of the lophophore, as it is in all lophophorates. This position is responsible for the alternative name "Ectoprocta" (= outside anus) for Bryozoa. Feces may be present in the rectum of the specimen under observation.
Note that the rectum and anus are dorsal, the mouth and lophophore are anterior, and the cecum is posterior. These are the best landmarks for recognizing the major body axes of these bilaterally symmetric animals. Find anterior, dorsal, posterior, ventral, right and left. Locate the plane of symmetry.
Figure 3. Adjacent zooids of Plumatella showing the digestive system. Drawn from a fixed and mounted specimen. A. View from the left. B. View from the right. Bryozoa89L.gif
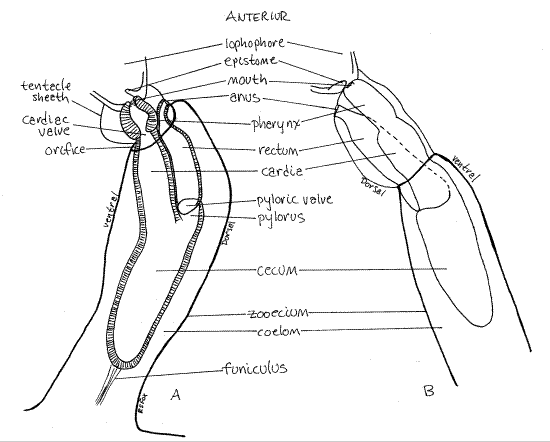
Funiculus
Use the highest power available with the dissecting microscope (40-50X) to examine the interior of a zooid (Fig 1, 3). Use transmitted light (substage light) to illuminate the interior. Remember that the space within the zooid is a coelomic space, specifically the metacoel.
Look carefully in the coelom for a transparent band of mesothelial tissue extending from the stomach posteriorly into the stolon where it attaches to the stolon wall (cystid). This is thefuniculus (Figs 1, 3, 25-16) and it is thought to be a through transport system that moves food from the gut to other parts of the zooid and stolon. The testis is located on the funiculus and is nourished by it. In phylactolaemates such as Plumatella the most conspicuous features associated with the funiculus are the numerous oval statoblasts in various stages of development (Fig 3). Statoblasts are produced asexually in the fall and are overwintering stages.
Musculature
Phylactolaemate bryozoans have unspecialized layers of circular and longitudinal body wall muscles and lack the specialized parietal muscles characteristic of gymnolaemates. The coelom is pressurized by contraction of the circular body wall muscles. Well-developed longitudinal muscles are present in the tentacles of all bryozoans and are used to bend the tentacles. You saw evidence of their presence if you observed tentacular flicking.
Two long lophophore retractor muscles extend from the posterior end of the zooecium to the base of the lophophore (Fig 3, 25-17). You watched them operate when you encouraged the animal to retract earlier in the exercise. Extension is accomplished by contraction of the circular muscles to pressurize the coelomic fluid.
Retracted Autozooid
1c. >.Briefly examine a retracted zooid. Although their orientation with respect to other organs has changed, you should be able to find the retracted lophophore, with its tightly clustered tentacles, and most of the regions of the gut. When the tentacles are withdrawn, they are enclosed by the retracted body wall (Fig 25-17). <
Reproduction
Phylactolaemates are hermaphroditic with the testis on the funiculus and the ovary on the endocyst of the ventral body wall. Self-fertilization is apparently the rule in phylactolaemates. Embryos are gestated in the trunk coelom and released as ciliated larva to settle on an appropriate substrate and found a new colony. The colony grows through the clonal formation of external buds, which in phylactolaemates occurs on the ventral body wall.
Statoblasts are internal buds produced clonally and used to survive winter conditions. Statoblasts are produced by adult zooids in the fall after which the colony dies and are capable of surviving freezing or desiccation. Although not independently motile, some statoblasts, especially those known as floatoblasts,are capable of being dispersed over great distances and are an effective dispersal stage for these otherwise stationary animals.
Statoblast morphology varies with species and is useful in species identification. Many species that cannot be distinguished by zooid anatomy can only be recognized by their statoblasts. Statoblasts are composed of two valves which together form an oval or circular disk with a thickened center and thin edges, reminiscent of Saturn and its rings or a fried egg (Fig 25-33, 3). The thickened center contains a clonal bud of undifferentiated, totipotent cells from which a new colony can develop in the spring. Statoblasts are produced on the funiculus or the endocyst mesothelium.
Two types of statoblasts, floatoblasts and sessoblasts, occur in phylactolaemates and Plumatella has both. Floatoblasts have gas-filled chambers in the thin periphery, and float, making them effective dissemules. Sessoblasts are attached to the substratum and are immobile. Spinoblasts are a type of floatoblasts with peripheral spines. Pectinatella has spinoblasts.
1d. > Examine a wholemount slide of Pectinatella statoblasts. These are floatoblasts, specifically spinoblasts. Note the overall shape of the statoblast. Find the central capsulewhich contains the undifferentiated cells. These cells are the only living parts of the statoblast. All the rest is secreted. An annulus of small gas chambers surrounds the capsule. In Pectinatella spinoblasts, bifid spines protrude from the periphery of the annulus. Make a labeled sketch of the statoblast.
References
Hyman LH. 1959. The Invertebrates. Smaller Coelomate Groups, V . McGraw-Hill, New York. 783 p.
Mukai H, Terakado K, Reed CG. 1997. Bryozoa, pp 45-206 in Harrison FW, Woollacott RM (eds), Microscopic anatomy of Invertebrates, vol 13. Lophophorates, Entoprocta, and Cycliophora. Wiley Liss, New York.
Pennak RW. Fresh-water invertebrates of the United States, 3 rd ed. Wiley Interscience, New York. 628 pp.
Ruppert EE, Fox RS, Barnes RB. 2004. Invertebrate Zoology, A functional evolutionary approach, 7 th ed. Brooks Cole Thomson, Belmont CA. 963 pp.
Ryland JL. 1970. Bryozoans. Hutchinson Univ. Press, London. 175 p.
Wood TS. 2001. Bryozoans, pp. 505-526 in Thorp JH, Covich AP (eds). Ecology and classification of North American freshwater invertebrates. Academic Press, San Diego.
Woollacott RM, Zimmer RL. 1977. Biology of Bryozoans. Academic Press, New York. 566 p.
Supplies
Dissecting microscope
Compound microscope
Living Plumatella or slides of colony fragments
Commercially prepared and stained wholemount slides of Plumatella
Commercially prepared and stained wholemount slides of Pectinatella
Slides, coverslips
Lake water (for living specimens)
8-cm culture dish
Carmine suspension
|
Slide |
Source |
|
Plumatella wholemount |
Carolina ? |
|
Pectinatella wholemount |
Ward’s, Triarch, |
|
Pectinatella statoblasts (spinoblasts) |
Ward’s |
|
FW bryozoan zooid (unspecified sp) |
Ward’s |