Invertebrate Anatomy OnLine
Pleurobrachia pileus ©
Comb Jelly
30jun2006
Copyright 2001 by
Richard Fox
Lander University
Preface
This is one of many exercises available from Invertebrate Anatomy OnLine , an Internet laboratory manual for courses in Invertebrate Zoology. Additional exercises can be accessed by clicking on the links to the left. A glossary and chapters on supplies and laboratory techniques are also available. Terminology and phylogeny used in these exercises correspond to usage in the Invertebrate Zoology textbook by Ruppert, Fox, and Barnes (2004). Hyphenated figure callouts refer to figures in the textbook. Callouts that are not hyphenated refer to figures embedded in the exercise. The glossary includes terms from this textbook as well as the laboratory exercises.
Systematics
Eumetazoa, Triploblastica, Ctenophora P, Cydippida O, Pleurobrachiidae F (Fig 8-15)
Introduction
Ctenophores, or comb jellies, are delicate, transparent, mostly pelagic, marine carnivores. They have biradial symmetry, oral-aboral axis of symmetry, and triploblastic organization with a thick cellular mesoglea. Some true organs are present. About 80 species, ranging in size from millimeters to over a meter, are known (Fig 8-1, 8-12, 8-13, 8-14).
Comb jellies are gelatinous zooplankters that superficially resemble scyphomedusae but differ from them in several important respects. There have no alternation of generations, no equivalent of the polyp generation, no dimorphism, and no colonial (modular) organization. Cnidocytes are absent and cilia, rather than muscles, are used for locomotion. The gut is complete, with openings at both ends. Ctenophores were long thought to be allied with cnidarians and included in the now defunct group Coelenterata. We now believe the similarities to be convergences and ctenophores are probably closer to Bilateria than to Cnidaria (Fig 8-15).
Digestion begins extracellularly but is completed intracellularly. The gut is divided into digestive and distributive regions similar to the situation in scyphozoans. Cilia are well developed and used for a variety of purposes. Locomotion is accomplished by eight longitudinal rows of paddles, each paddle being composed of thousands of cilia. Cilia are also involved in sensory reception and some function in a mechanical, rather than neuronal, communication system. The connective tissue compartment is a mesoglea containing cells of several types, especially muscle. Ctenophores are hermaphroditic (unlike scyphomedusae) and many are bioluminescent.
Laboratory Specimens
Ctenophores should be studied alive if possible but preserved material must be used in most laboratories. Because of their delicacy, living ctenophores are difficult to ship and their study is a luxury possible only at coastal laboratories. Observation of living comb jellies is an altogether different experience than the study of preserved animals.
Cydippid ctenophores, such as the sea gooseberry Pleurobrachia (Fig 1, 8-1B), are more or less typical. Pleurobrachia pileus is the species usually provided in preserved condition by biological supply companies. It is a small animal, about 1 cm in diameter.
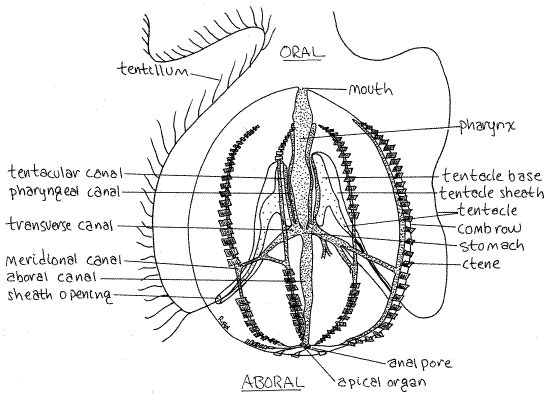
Figure 1. The comb jelly, Pleurobrachia pileus, drawn from a preserved specimen. The tentacular plane coincides with the plane of the paper (or screen). The pharyngeal plane is perpendicular to it. The tentacles are shown extended, as they would be in life but not when preserved. Some ctenes have been omitted to reveal structures beneath them. Cten12L.gif
External Anatomy
General
Place a preserved specimen in a small dish of tapwater {or isotonic magnesium chloride if living}. Examine it with the dissecting microscope. If your specimen is transparent, you will find transmitted light preferable to incident. Handle your specimen carefully as it is delicate and easily broken.
The ctenophore body wall consists of a thin, bilayered, outer epidermis and a thin inner gastrodermis lining the coelenteron. Between the two is the thick, gelatinous, cellular, collagenous mesoglea.
Symmetry
Pleurobrachia is a slightly ovoid sphere weakly flattened on two opposite sides. The two ends of the ovoid are the oral and aboral poles (Fig 1, 8-1A). In preserved material the aboral pole is often recessed in a depression whereas the oral pole may protrude somewhat. The mouth is a transverse slit at the oral pole. In preserved specimens it may be elevated above the body surface by the partial eversion of the pharynx. Two anal pores are present at the aboral pole.
Figure 2. Aboral polar view of the cydippid larva of an unidentified ctenophore (probably Mnemiopsis) from Beaufort, North Carolina. The diameter is about 0.5 mm. cten13L.gif
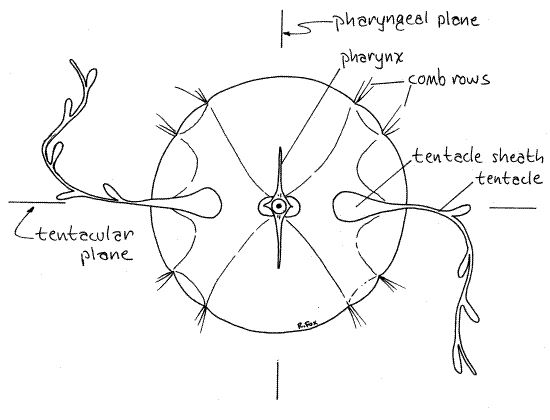
The oral-aboral axis is the axis of symmetry around which is arrayed the biradial symmetry characteristic of ctenophores. Two planes, both including the axis of symmetry and at right angles to each other, are the planes of symmetry (Fig 2). One, the tentacular plane, passes through the two tentacle sheaths on opposite sides of the body. Another, the pharyngeal plane, passes through and includes the plane of the flattened pharynx. These two planes, and no other, divide the jelly into equal, mirror image halves.
Comb Rows
The most conspicuous features of a ctenophore are its eight comb rows, which are arranged like meridians, or lines of longitude, on the surface of the sphere (Fig 1, 8-3, 8-2). Each row is composed of a number of successive plates of large, fused cilia. Each plate functions as a paddle and is known as a ctene (= comb).
Tentacles
The two tentacle sheaths (Fig 1, 8-9) are deep ectodermal invaginations opening on the surface of the aboral hemisphere. They extend into the interior to terminate on opposite sides of the pharyngeal region of the gut. The sheaths and tentacles lie in the tentacular plane of the animal.
A single tentacle arises from a large, opaque tentacle bulb (=tentacle base) at the bottom of each sheath. The tentacles extend from the sheath and trail through the water where they fish for zooplankton. The tentacles are branched, unlike those of cnidarians, and the branches, known as tentilla, bear colloblasts. Colloblasts are cells reminiscent of cnidocytes and, like them, arise from epidermal interstitial cells. They release mucus rather than toxins when discharged onto the prey and they do not penetrate.
The tentacles are muscular and can be retracted into the sheaths. Completely extended tentacles of Pleurobrachia may be 25 cm in length but in preserved specimens the tentacles are partly or completely retracted.
Coelenteron
The mouth opens into a large, wide, flat, epidermal pharynx where extracellular digestion begins. The pharynx is strongly flattened in the pharyngeal plane.
The pharynx extends aborally from the mouth and empties into the small stomach near the center of the animal (Fig 1, 8-9). Unlike the pharynx, the coelenteron, including the stomach, is endodermal (i.e. gastrodermal) and is lined with ciliated, secretory, phagocytic gastrodermis.
An extensive system of ciliated endodermal canals arises from the stomach and distributes food to the tissues (Fig 1, 8-9). It includes eight meridional canals (one below each comb row), two tentacular canals (one to each tentacle sheath), two pharyngeal canals to opposite sides of the pharynx, a pair of transverse canals, and an aboral canal extending vertically to open to the exterior via two anal pores at the aboral pole. Notice that all metabolically active tissues have a canal in close proximity.
As is the case in Scyphozoa and Anthozoa, the gut is divided into a central digestive region where extracellular digestion occurs and a peripheral distributive portion. The pharynx is the major extracellular digestive chamber and the canals are the distribution, or fluid transport, system. The gastrodermis lining the distributive canals is composed of both ciliated and phagocytic cells. Extracellular digestion begins in the pharynx with the secretion of hydrolytic enzymes. Partially digested food moves into the stomach and from there into the canal system, propelled by ciliary currents. In the canals the partially digested material is phagocytized or pinocytosed (endocytosed) and digestion is completed intracellularly. Undigested material passes through the aboral canal and is voided through one of the two anal pores at the aboral pole.
The epithelial walls of the canals include cells specialized for light production (bioluminescent photocytes), osmoregulation (rosettes), endocytosis and intracellular digestion, gametogenesis (gonads), and fluid transport (monociliated cells).
> {If you have a living specimen, inject a dye solution, such as 1% toluidine blue in sea water, into the mouth and pharynx to visualize them. This works much better in living animals because the gastrodermal cilia assist in the distribution of the dye. The results can be spectacular.} <
" If the body wall is opaque and obscures your view of the interior, your instructor may direct you to dissect the animal. Do this by cutting from pole to pole through the epidermis on one side with fine scissors. Look into the interior through the cut. The pharynx, tentacle sheaths, and tentacles should be easier to see now. Relocate these structures and look once more for the canals of the gastrovascular cavity. "
Ctenes
" Remove a small piece of a comb row and make a wet mount with it. Your instructor may designate a damages specimen for use by several students for this purpose. Examine the wetmount with the compound microscope. Find a ctene and note its composition. Look for the individual cilia of which it is composed. Do you understand now why these plates are called "combs"?
Aboral Organ
The aboral organ (= apical organ) can be seen as a small spot at the aboral pole (Fig 1, 8-4). It is a center for gravity detection and control of the comb rows. It contains a calcareousstatolith, which is easily seen with magnification (Fig 8-7). The statolith rests on four tufts of balancer cilia (Fig 8-4B). From each balancer run two ciliary grooves, one each to the aboral ends of each pair of side-by side comb rows. Tilting the body away from its vertical orientation increases the pressure of the statolith on one or two of the balancers. Pressure of the statolith on a balancer changes the beating rate of its cilia. This change is transmitted mechanically, not by neurons, to the cilia of the ciliary groove and thence to the cilia of the ctenes. The ctenes of those comb rows beat faster and return the animal to vertical.
" If instructed to do so, use fine scissors to excise the aboral pole being sure to include the aboral organ. Carefully arrange the apical organ on a slide and prepare a wetmount. Be sure the epidermis is not folded over the apical organ.
Look for the statolith, four balancer tufts of cilia upon which the statolith rests, and a ciliated groove extending to each comb row. This is the apparatus that controls the beating of the ctenes in the comb rows.
>Test the composition of the statolith by drawing 8% HCl (See Techniques chapter) under the coverslip. Watch the response of the statolith. If it is calcareous, it will react with the HCl, release carbon dioxide, and disappear. <
Reproduction and Development
Ctenophores are hermaphroditic and gonads of both sexes are located in the lining of the meridional canals but they are usually not evident in preserved specimens (Fig 8-4A). Gametes are shed to the sea through numerous tiny gonopores in the comb rows. In most species fertilization is external.
Development is direct without metamorphosis but includes a characteristic cydippid larva (Fig 2) which resembles the adult (Fig 8-11).
*Hyphenated call-outs, such as this one, refer to figures in Ruppert, Fox, and Barnes (2004). Those without hyphenation refer to figures embedded in this exercise.
References
Hernandez-Nicaise, M-L. 1991. Ctenophora, pp 359-418 in Harrison, F. W. & J. A. Westfall (eds.). 1991. Microscopic Anatomy of Invertebrates vol. 2 Placozoa, Porifera, Cnidaria, and Ctenophora. Wiley-Liss, New York. 436p.
Horridge, G. A. 1965. Relations between nerves and cilia in ctenophores. Am. Zool. 5:357-375.
Horridge, G. A. 1974. Recent studies on the Ctenophora. in Muscatine, L. and H.M. Lenhoff (eds) Coelenterate Biology. Academic Press, New York. pp. 439-468.
Mayer, A. G. 1912. Ctenophores of the Atlantic coast of North America. Carnegie Inst. Washington Pub 162:1-58, 17 pls.
Ruppert EE, Fox RS, Barnes RB. 2004. Invertebrate Zoology, A functional evolutionary approach, 7 th ed. Brooks Cole Thomson, Belmont CA. 963 pp.
Supplies
Dissecting microscope
Preserved Pleurobrachia
Small culture dish
Dropper bottle of 8 % hydrochloric acid
1 % toluidine blue