Invertebrate Anatomy OnLine
Introduction ©
2jul2006
Copyright 2001 by
Richard Fox
Lander University
Preface
This is the introductory chapter from Invertebrate Anatomy OnLine , an Internet laboratory manual for courses in Invertebrate Zoology. Additional exercises can be accessed by clicking on the links to the left. Terminology and phylogeny used in these exercises correspond to usage in the Invertebrate Zoology textbook by Ruppert, Fox, and Barnes (2004).
Introduction
This OnLine Laboratory Manual is a collection of exercises to assist you in your study of invertebrate animals. The exercises emphasize morphology but include occasional opportunities to study behavior or physiology. The use of living or fresh, as opposed to preserved, specimens is encouraged although providing such material is often not practical. The collection includes most of the species in the traditional Invertebrate Zoology repertory and in addition, many additional ones selected because they are readily and inexpensively available alive. Most of the exercises are written for use with fresh specimens but can be used with preserved material when necessary.
It is often in the laboratory that relationships between structure and function first begin to make sense and the facts learned in lecture become meaningful. It is the laboratory in Invertebrate Zoology that brings the course to life.
These exercises are keyed to the invertebrate zoology textbook by Ruppert, Fox, and Barnes (2004) and follow it in terminology, classification, and style. Each exercise contains, in addition to it own figures, callouts to illustrations in this text.
A few conventions and abbreviations are used throughout the manual. Words in bold type refer to structures you are expected to locate and/or know. The term appears in bold at the time it is appropriate to locate the structure in the specimen, not necessarily the first time it is used. A single structure may appear in bold more than once if you are to find it a second time.
> Small arrowheads set off supplemental information and exercises which may be undertaken at the discretion of the instructor. <
(Parenthetical comments usually point out differences between similar species.)
{Statements in brackets apply to living organisms.}
Systematics
A classification of each species is given at the beginning of its section to help you understand the evolutionary relationships between species. Access to this information can lead to insights about the significance of similarities and differences between species and groups. It is not intended that you memorize the classification. Although modern biology has moved away from traditional Linnean classification many biologists are comfortable with it and prefer it to cladistic classifications. Accordingly where appropriate the Linnaean category is indicated as a superscript abbreviation following the taxon name. Abbreviations used are: P = phylum, C = class, O = order, F = family, s = sub, S = super, i = infra. To help you understand the phylogenetic relationships of each taxon callouts to phylogenetic trees (cladograms) in Ruppert, Fox, and Barnes (2004) are provided.
Terminology
Terminology of anatomic features among invertebrate taxa has been standardized whenever possible to reduce the multiplicity of names for the same structure. Where similar structures in different taxa have in the past been assigned different names, an attempt has been made to use the same name. The terminology used here conforms with that of Ruppert, Fox, Barnes (2004) and continues the standardization initiated by that text.
Symmetry
Radial Symmetry
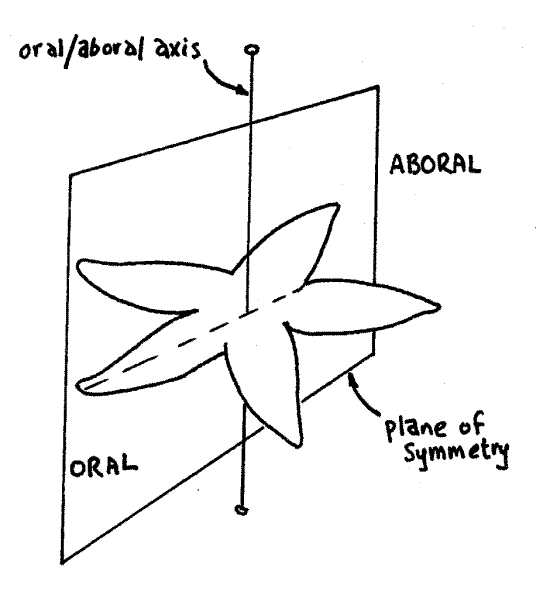
The two major types of symmetry among metazoan animals are radial and bilateral. Radial symmetry is associated with sedentary, immobile, or slow-moving animals. A typical radially symmetrical animal has no preferred direction of motion and can meet its environment equally well in any direction. Sense organs are not concentrated in any preferred area nor is the animal streamlined for maximum efficiency of motion in a preferred direction. Cnidarians and echinoderms are the major radially symmetrical phyla.
A radially symmetrical animal usually has a mouth at or near the center of one surface, which is consequently known as the oral surface (Fig 1). The opposite surface is the aboral surface and it may, or may not, bear an anus. The oral/aboral axis is a line connecting the center of the oral and aboral surfaces. It is the animal's major axis and is the axis of symmetry. Any plane of symmetry must include this axis. A plane of symmetry is any plane that divides an object into two equal, mirror-image halves.
Radial animals, by definition, have multiple planes of symmetry. That is, there are several ways to divide the animal into equal halves. In Figure 1, the plane illustrated is one of five possible planes of symmetry. Each of them includes the aboral-oral axis, which is the axis of symmetry. This particular example is of pentamerous radial symmetry because there are five planes of symmetry. Pentamerous radial symmetry is typical of echinoderms. Tetramerous radial symmetry is also common, especially among cnidarians.
A radially symmetrical animal has no head, tail, front, back, right, or left and the terms anterior, posterior, dorsal, ventral, lateral, and medial are inappropriate. Structures near the axis of symmetry are referred to as central whereas those away from it are peripheral.
Adult echinoderms are said to possess secondary radial symmetry because they evolved from ancestors that were bilaterally symmetrical. Cnidarians, on the other hand, have primaryradial symmetry since their ancestors were not bilaterally symmetrical.
Figure 1. View of the aboral surface of a sea star as an example of radial symmetry. This animal has five planes of symmetry, of which one is illustrated. Drawing by John Norton. lab1L.gif
Bilateral Symmetry
Bilaterally symmetrical animals typically are mobile (or derived from mobile ancestors) and have a preferred orientation for meeting and moving through their environment. The sense organs and nervous integration and coordination center are concentrated at the end that first encounters the new environment, i.e., the head. The animal is streamlined to maximize efficiency of locomotion with the head foremost.
The end with the head is the anterior end (Fig 2). The opposite end, or tail, is the posterior end. The top, or back, of the animal is the dorsal surface, or dorsum, and the lower surface, or belly, is the ventral surface, or venter.
The axis of symmetry of a bilaterally symmetrical animal is the anterior/posterior axis. By definition, a bilaterally symmetrical animal has only one plane of symmetry. It is the vertical plane that divides the animal intoright and left mirror images.
Structures near the plane of symmetry (i.e., the center of the animal) are said to be median, or medial, whereas those away from the center and near the sides are lateral. The human nose, for example, is a median structure whereas the ears are lateral. Structures close to the center of the body are referred to as proximal whereas those closer to the periphery are distal. The terms are relative and are used when making comparisons. For example, the elbow is distal to the shoulder but it is proximal to the hand.
The imaginary line running along the middle of the dorsum from head to tail is the dorsal midline, or middorsal line. A similar line along the middle of the venter is the ventral midline, or midventral line. These lines mark the intersection of the plane of symmetry with the body surface.
Figure 2. View of the left side of a bilaterally symmetrical animal. Drawing by John Norton. lab2L.gif
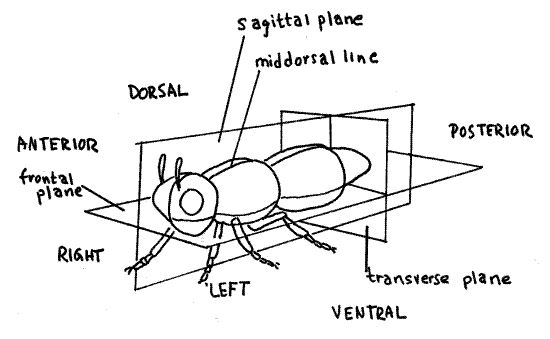
Three different types of planes pass through a bilaterally symmetrical animal at right angles to each other. Any vertical plane parallel to or coinciding with the plane of symmetry is asagittal plane (Fig 2). The plane of symmetry is the median sagittal plane, or midsagittal plane, and includes the dorsal and ventral midlines. A frontal plane is parallel to or includes the axis of symmetry but is at right angles to the sagittal plane. A frontal plane is horizontal and divides the animal into dorsal and ventral portions but never into mirror images. Transverse planes are perpendicular to the sagittal and frontal planes and pass across the animal, dividing it into anterior and posterior portions. They are perpendicular to the anterior/posterior axis and never include it.
A cut made through an organism is known as a section. A cut coinciding with a sagittal plane is a sagittal section, one coinciding with a frontal plane is a frontal section, and one with a transverse plane is a transverse section. Transverse sections are also known as cross sections because they cut across the long axis of the animal. Both sagittal and frontal sections are types of longitudinal sections because they cut the animal lengthwise, parallel to its long axis.
Observational Records
Students in the sciences should develop the habit of keeping accurate, up to date, and informative records of observations. It is a mistake to rely on memory for recollections of even the most impressive events fade rapidly and become unreliable.
This manual is a guide to help you observe animals and you are encouraged to keep a loose-leaf notebook of notes and drawings that you think are important. Learn to recognize important and noteworthy information.
Note taking and drawing can be very time-consuming so it is important you learn to recognize information worth the effort. Because it requires so much time, your instructor may prefer that you not make drawings.
The primary objective of drawings is to render an accurate image of the organism or structure under observation thereby providing a reliable record for future reference. Drawing may be difficult for you at first but, regardless of talent, you should be able to execute useful drawings by the end of the course.
First make a general inspection of the object to be drawn and plan the layout of your drawing. The drawing should fit comfortably in the space available on the paper. You may need to enlarge or reduce it to fit it onto the page. It should not crowd the space nor should it be lost in it. Be sure to save room for labels. Drawings should always be made in pencil.
Many biologists like to sketch in the major axes or make a faint outline before beginning. Indicate very lightly the position and shape of the major parts of the object to insure that their final position and relative size is correct. These faint pencil marks are called helping lines and they will be erased after the drawing is completed.
Make the final drawing using firm, strong, solid lines made with a sharp, hard pencil. Avoid hesitant, timid, sketchy, or fuzzy lines. Make sure all lines connect with something and that the connections are clear and distinct. Avoid hiding your confusion about relationships in a tangle of lines.
Label the drawing carefully and completely. Be sure they are written horizontally. Connect the labels with the appropriate structure in the drawing with a ruled straight line. Do not use arrowheads at the end of the lines. Do not let any of the lines cross each other on the way to the object.
Label the entire drawing at the center of the top or bottom of the drawing. For microscopic animals include an indication of the magnification at which the drawing was made.
Keep in mind that your goal is to produce an accurate record of your observations, not simply to render an impression of an object's appearance. Consequently, you should draw what you see and should not adopt conventions or symbols that represent structures without depicting them accurately.
Shading and the use of colored pencils is usually discouraged but your professor may instruct you otherwise. It is best to make simple outlines of structures and without coloring or shading. Shading by beginners usually confuses the image and detracts, rather than adds, information.
If you wish you may stipple structures to emphasize differences. If you choose to stipple, do so carefully, making each small dot individually. Stipples of different sizes and spacing can be used for additional contrast.
Dissection
Invertebrate zoology is one of the few opportunities in the modern undergraduate biology curriculum to develop the skills needed to conduct a firsthand study of animal morphology.
Microdissection
Most of the dissections in this course are of animals less than 10 cm in length and will usually be conducted on the stage of a dissecting microscope. Don't be intimidated by the prospect of conducting dissections under magnification. The experience is an opportunity to learn an important skill that may later prove useful in courses or careers in animal development, physiology, or medicine.
Working under a dissecting microscope will at first seem awkward and you will feel clumsy and uncoordinated as you watch the tremors of your hand, magnified 30 times or so, send the tips of your forceps or needles flying across the field to positions you never intended. The first dissection or two may be frustrating but you will soon learn the necessary tricks and acquire the motor control and coordination required. Microdissection is neat, precise, and amenable to variable magnification and you may come to prefer it to macrodissection.
Dissecting Technique
In general, the object of any dissection, whether of a large or small animal, is to expose tissues, organs, and organ systems in their natural condition and with their natural relationships intact. Realize that the tissues, especially those of living animals, are very delicate so you must develop the habit of handling them carefully. Avoid making random, vague, and unnecessary movements with your tools and do not introduce a tool into the specimen unless you have a specific task for it.
Contrary to a common misconception, good dissection involves minimal cutting. Instead, you should strive to expose structures intact, without cutting them or nearby tissues, whenever possible. Most dissection consists of separating the tissue around organs to free it from nearby structures using teasing needles, forceps, and a blunt probe. When you are finished, you should be able to see clearly the organ of interest and perhaps lift it and look at all its surfaces. The organ can be moved aside (reflected) to reveal deeper organs but can be returned to its original position when desired. A good dissection reveals, accentuates, and clarifies, but does not alter, the natural spatial relationships between structures.
The blunt probe in your dissecting kit is one of its most useful instruments. Its major use is to manipulate structures, move organs aside, or lift them. It is ideal for separating adjacent organs as it is least likely to puncture or tear delicate tissues. It is especially useful for tracing channels and passageways. Its tip should not be over 1 mm in diameter.
The scalpel is the favorite instrument of beginning students, perhaps because it symbolizes the glamour of surgery. In reality it is the least important tool in your kit and the one with which you can do the most damage to the natural shape and relationships of the animal. It is a very specialized tool with specific functions. Neither of your two cutting instruments, scissors and scalpel, should be used often, but when cutting is necessary, scissors are almost always the tool of choice. With scissors you have greater control over direction and depth of cut than with the scalpel. In addition, scissors provide their own firm cutting surface whereas a scalpel pushes soft, unsupported tissue out of its way instead of cutting it. Never use the scalpel as a probe or pointer.
When it is necessary to cut an organ in order to expose underlying structures, it should not be removed entirely. Instead, use needles and forceps to free the superficial organ from the surrounding tissues. Once it is free, lift it in its middle and cut cleanly across it with the scissors. The two opposite ends will retain their natural connections with the body but can be reflected to expose deeper tissues. When it becomes desirable to see the superficial organ again in its original condition, the two ends can be moved back into place.
In any dissection the directions “right” and “left” ALWAYS refer to the animal's right and left. This may or may not correspond to your own right and left depending on your orientation with respect to the specimen.
In almost all cases specimens should be dissected under liquid rather than dry. The liquid should completely cover the specimen. This supports the tissues, prevents desiccation, and eliminates reflections from glistening surfaces. Obviously, the fluid should be appropriate to the circumstances. Living or fresh marine animals should be in seawater or whatever relaxant, usually isotonic magnesium chloride, is used to anesthetize them. Living freshwater and terrestrial animals should be in tapwater, relaxant, or isotonic saline. Preserved specimens should be in tapwater.
Dissecting Tools
The dissecting set for invertebrate zoology should contain the standard instruments found in high quality sets used in freshman biology or comparative vertebrate anatomy plus a few tools suitable for small specimens. The tools should be stainless steel when possible. The standard dissecting set should include:
1 pr 6" surgical scissors with one blunt, one pointed blade
1 pr heavy forceps
1 pr fine forceps
1 blunt probe, the finer the tip the better
1 # 3 scalpel handle with disposable # 10 blades. (Numbers 11, 12, 15
also fit the # 3 handle.)
1 straight teasing needle with wooden or plastic handle
1 bent teasing needle with wooden or plastic handle
1 15 cm plastic rule
1 plastic or wooden box to contain the tools
In addition you will need the following:
1pr fine-pointed watchmaker's or microdissection forceps
1 pr iridectomy scissors
2 minuten nadeln on applicator sticks, one with straight point, one
bent
2 cloth towels
1 pkg #1 insect pins, preferably stainless steel
The minuten nadeln on sticks will be provided by the teaching staff or you may make them yourself during an early laboratory session using instructions in the Techniques Chapter.
The microdissecting forceps are expensive and delicate and should be handled carefully. The points must be protected from damage and you should make every effort to avoid dropping them. With the tips open, push them into a #1 cork. The forceps should always be stored with the cork in place.
The iridectomy, or iris, scissors are fine scissors with spring handles. They are closed by pinching the handles together and they open by themselves when pressure on the handles is relaxed. They are used for fine, controlled cutting as their name suggests. These may be provided by the laboratory or you may be expected to purchase your own. More than any other tool in your kit, they require careful cleaning and drying after each use. It is particularly important that the pin holding the two blades together be dry. They should be thoroughly dried with a towel and then dipped in acetone and dried again after each session. The slightest bit of rust or corrosion between the blades with prevent them from opening.
Your tools should be washed with warm water, rinsed, and dried thoroughly after each use. Properly cared for, a dissecting set with high quality tools will easily last through your student years and beyond.
Absorbent terrycloth towels are useful in the laboratory. A towel spread flat beside the dissecting microscope serves as a staging area for wet dishes before they are placed on the microscope. It will absorb any liquid, such as seawater or magnesium chloride and prevent its coming in contact with the microscope.
Before beginning work, remove all your tools from their case and array them on the towel ready for use. Another towel can be used to dry your hands and dissecting tools.
Specimens
For several reasons, living anesthetized animals are, in almost all cases, incomparably preferable to preserved specimens. Living animals must always be treated humanely and should be relaxed or anesthetized prior to dissection.
Living specimens provide the opportunity to study the behavior and physiology of the organism before the dissection begins. They are usually easier to dissect because their muscles are not contracted and the shape is not distorted. Living specimens are often distinctly and usefully "color coded" with organs of different colors making it much easier to recognize and identify them. Cilia continue to beat in relaxed, living specimens making it possible to observe ciliary respiratory currents, food tracts, fluid transport patterns, sperm activity, filter feeding mechanisms, and coelomic circulation. It is often possible to observe the beating of the heart and peristalsis of the gut, body wall, and blood vessels. Using living specimens facilitates impromptu, unscheduled investigations of any questions arising during a dissection.
The use of fresh specimens makes the study of animals exciting and dynamic. Students invariably enjoy such dissection even though they may dislike standard dissections of preserved material. With living material there is the likelihood you will see something your instructor has never seen.
Anesthetization and Relaxation
Anesthetization renders the animal unable to feel pain whereas relaxation refers to the condition of the muscles. Many agents do both simultaneously and in general most anesthetics also cause muscle relaxation. For humane reasons it is necessary that the animal be anesthetized but relaxation is done to make the dissection easier and more instructive. For marine animals anesthetization and relaxation is usually accomplished using magnesium chloride or magnesium sulfate isotonic with the seawater inhabited by the animal. This is referred to throughout the text as "isotonic magnesium chloride". The magnesium ion interferes with the movement of calcium across the sarcoplasmic reticulum and the presynaptic membrane thereby inactivating muscular and nervous systems. It does not interfere with cilia or flagella.
Most terrestrial arthropods can be anesthetized with fumes of chloroform or ether or killed with ethyl acetate fumes. Carbon dioxide, either as a gas for terrestrial animals or in solution as carbonated water for aquatic animals is an excellent anesthetic.
Ethanol (5%), chloretone (0.2%), carbonated water (club soda), and chloroform-saturated water are the most widely used anesthetics for freshwater animals.
Acknowledgements
The assistance of colleagues and friends has been essential for the construction of this collection. The late Dr. Charles Jenner of the University of North Carolina at Chapel Hill kindled in me, as he did in so many others, an enduring interest in invertebrates.
I thank friends and colleagues who tested early drafts of these exercises in their invertebrate zoology laboratories. Among these are Dr. Edward Ruppert of Clemson University, Dr. Steve Stancyk of the University of South Carolina, Dr. A. Quinton White of Jacksonville University, and Dr. Nora Terwilliger of the University of Oregon Institute of Marine Biology. Their comments have contributed importantly to the final form of the manual.
I am grateful to the faculty, staff, and administration of Lander University for the support, laboratory facilities, faculty development grants, and sabbatical leave that contributed to the development of this manual.
I appreciate the efforts of my students who used these exercises, usually in early form and without benefit of illustrations, in my classes at Lander University, the Duke University Marine Laboratory, and the Harbor Branch Oceanographic Institution. It truly would not have been possible to produce this manual without their feedback. I thank Lisa Martin, whose senior honors project at Lander University contributed importantly to my understanding of the nervous system of the apple snail, Pomacea paludosa. Dr. Laura Corley, also a former honors student and currently on the faculty at Washington State University, helped me understand ascidian anatomy. Dave Dulaney discovered and brought to my attention the suctorians, sponges, and tardigrades on floating docks in Lake Greenwood.
Gathering information for this manual gave me an excuse to spending summers and holidays at marine laboratories on both coasts under the guise of working. I am thankful for the hospitality shown me by the directors, faculty, and students of these labs. Among them are Dr. Dennis Allen of the University of South Carolina Baruch Field Laboratory, Dr. John Costlow of the Duke University Marine Lab, Dr. Charles Peterson of the University of North Carolina Institute of Marine Science, Dr. Les Watling, Dr. Bernie McAlice, Dr. David Dean, Tim Miller, Rick Wahle, Chris Chambers, and Linda Kindblom made my stay the University of Maine Darling Center for Research, Teaching, and Service a profitable one. Tim Miller and Linda Kindblom provided me with most of the animals I needed for my work in the northeast. Dr. Jan Hodding, Dr. Nora Terwilliger, Ronnie Estelle, and Chad Hewitt of the Oregon Institute of Marine Biology in Charleston introduced me to the Oregon coast, provided laboratory space, and helped me find invertebrates. Dr. Kevin Eckelbarger, John Miller, and Paula Mikkelson welcomed me to Harbor Branch Oceanographic Institution in Ft. Pierce and provided me with laboratory space, boat time, and assistance.
Many colleagues helped with animals they know particularly well. Dr. Edward Ruppert provided expertise on echinoderms, tunicates, hemichordates, and nemerteans. The Glycera, Molgula, and Saccoglossus exercises were inspired by handouts he developed for use in his Invertebrate Zoology course at Clemson University. Dr. Elizabeth Balser, another of my former honors students and currently at of Illinois Wesleyan University helped with echinoids, crinoids, and acorn worms and as my teaching assistant supervised the testing of early drafts by students. Dr. Joseph Woodring, of the Louisiana State University was a valuable source of information about cricket anatomy and physiology. Dr. Paula Mikkelson, former Curator of the Harbor Branch Oceanographic Museum in Ft. Pierce and currently at the American Museum of Natural History, provided me with an unpublished bibliography of the Florida apple snail, Pomacea paludosa, and supplied living snails for study.
Carolina Biological Supply Co. generously provided, at no cost, the prepared slides, preserved specimens, and cultures needed to prepare many of the exercises. Leeches USA supplied living medicinal leeches. Mr. John Valois of the Marine Biological Laboratory at Woods Hole, Massachusetts supplied living invertebrates at no charge.
Most of the illustrations are original and are based on pencil drawings of living or preserved organisms.
References
Ruppert EE, Fox RS, Barnes RB. 2004. Invertebrate Zoology, A functional evolutionary approach, 7 th ed. Brooks Cole Thomson, Belmont CA. 963 pp.