Invertebrate Anatomy OnLine
Cyclocephala
Scarabaeid Beetle Larvae ©
White Grubs
30jun2006
Copyright 2004 by
Richard Fox
Lander University
Preface
This is an exercise from Invertebrate Anatomy OnLine , an Internet laboratory manual for courses in Invertebrate Zoology. Additional exercises can be accessed by clicking on the links to the left. A glossary and chapters on supplies and laboratory techniques are also available. Terminology and phylogeny used in these exercises correspond to usage in the Invertebrate Zoology textbook by Ruppert, Fox, and Barnes (2004). Hyphenated figure callouts refer to figures in the textbook. Callouts that are not hyphenated refer to figures embedded in the exercise. The glossary includes terms from this textbook as well as the laboratory exercises.
Systematics
Arthropoda P, Mandibulata sP, Tracheata, Hexapoda SC, Insecta C, Dicondylia, Pterygota, Metapterygota, Neoptera, Eumetabola, Holometabola, Coleoptera O, Polyphaga sO, Scarabaeoidea SF, Scarabaeidae F, Rutelinae sF (Fig 16-15, 20-14, 20-15, 21-23)
Arthropoda P
Arthropoda, by far the largest and most diverse animal taxon, includes chelicerates, insects, myriapods, and crustaceans as well as many extinct taxa. The body is segmented and primitively bears a pair of jointed appendages on each segment. The epidermis secretes a complex cuticular exoskeleton which must be molted to permit increase in size. Extant arthropods exhibit regional specialization in the structure and function of segments and appendages. The body is typically divided into a head and trunk, of which the trunk is often itself divided into thorax and abdomen.
The gut consists of foregut, midgut, and hindgut and extends the length of the body from anterior mouth to posterior anus. Foregut and hindgut are epidermal invaginations, being derived from the embryonic stomodeum and proctodeum respectively, and are lined by cuticle, as are all epidermal surfaces. The midgut is endodermal and is responsible for most enzyme secretion, hydrolysis, and absorption.
The coelom is reduced to small spaces associated with the gonads and kidney. The functional body cavity is a spacious hemocoel divided by a horizontal diaphragm into a dorsal pericardial sinus and a much larger perivisceral sinus. Sometimes there is a small ventral perineural sinus surrounding the ventral nerve cord.
The hemal system includes a dorsal, contractile, tubular, ostiate heart that pumps blood to and from the hemocoel. Excretory organs vary with taxon and include Malpighian tubules, saccate nephridia, and nephrocytes. Respiratory organs also vary with taxon and include many types of gills, book lungs, and tracheae.
The nervous system consists of a dorsal, anterior brain of two or three pairs of ganglia, circumenteric connectives, and a paired ventral nerve cord with segmental ganglia and segmental peripheral nerves. Various degrees of condensation and cephalization are found in different taxa.
Development is derived with centrolecithal eggs and superficial cleavage. There is frequently a larva although development is direct in many. Juveniles pass through a series of instars separated by molts until reaching the adult size and reproductive condition. At this time molting and growth may cease or continue, depending on taxon.
Mandibulata sP
Mandibulata includes arthropods in which the third head segment bears a pair of mandibles. As currently conceived this taxon includes myriapods, hexapods, and crustaceans. Appendages may be uni- or biramous and habitats include marine, freshwater, terrestrial, and aerial.
Tracheata
Myriapods and hexapods share tracheae and a single pair of antennae and are sister taxa in Tracheata. Crustaceans, which have gills and lack tracheae, are excluded and form the sister group.
Hexapoda SC
The body is divided into three tagmata; head, thorax, and abdomen. Appendages are uniramous and a single pair of antennae is present. Three pairs of legs and two pairs of wings are found on the thorax of most adults. Hexapod legs are uniramous although there is increasing evidence that they evolved from multiramous appendages of their ancestors. Gas exchange is accomplished by trachea. Excretory organs are Malpighian tubules and the end product of nitrogen metabolism is uric acid. There is relatively little cephalization of the nervous system. Insects are gonochoric with copulation and internal fertilization.
Insecta C
Most hexapods are insects. A few hexapod taxa (orders) lack wings and have primitive mouthparts recessed into the head and belong to Entognatha, the sister taxon of Insecta. Insects have ectognath mouthparts and the adults (imagoes) of most taxa have wings.
Pterygota
The winged insects. These insects are derived from a winged common ancestor. Adults of most taxa have wings although they have been lost in some.
Eumetabola
Juveniles have no ocelli and there are six or fewer Malpighian tubules.
Holometabola
The final larval instar pupates and undergoes a radical metamorphosis in which it is converted to an imago, or adult. The imago is sexually mature and in most taxa has wings whereas larvae are immature and wingless. During metamorphosis many or most larval tissues are dismantled and adult structures built anew. Wings, for example, are manufactured from clusters of undifferentiated cells known as imaginal discs but not from preformed wingpads as in pauro- and hemimetabolous insects.
Coleoptera O
Coleoptera is the largest taxon (of equivalent rank) of organisms with about 300,000 described Recent species. The body of most beetles is heavily sclerotized. The forewings are sclerotized elytra, or wing covers. Beetle larvae are known as grubs and do not resemble adults.
Natural History
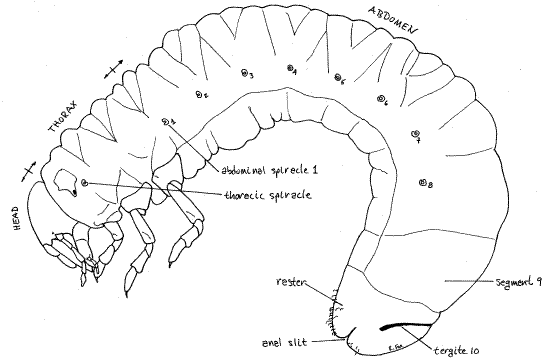
The larvae of many species of scarab beetles inhabit turf grasses and are collectively known as white grubs. They are similar to each other, white or grey, with a darker posterior abdomen, and brown head capsule. They curl into a characteristic C-shape (Fig 1, 21-14).
Figure 1. A Cyclocephala (masked chafer) grub viewed from the left side. Beetle71L.gif
Some common species of white grubs in the eastern United States are Japanese beetle (Popillia japonica), northern masked chafer (Cyclocephala borealis), southern masked chafer (Cyclocephala lurida), green June beetle (Cotinus nitida), May-June Beetle (Phyllophaga fusca), European chafer (Rhizotrogus majalis), Asiatic garden beetle (Maladera castanea), Oriental beetle (Exomala orientalis), false Japanese beetle (Strigoderma arbicola), and black turfgrass ataenius (Ataenius spretulus). The subterranean larvae feed on the roots of turf grasses and other plants whereas the adults consume the foliage of a variety of trees and shrubs. These grubs are distinguished from each other by the shape of the anus and pattern of spines on the venter of the tenth (last) abdominal segment.
The descriptions and drawings for this exercise are based on observations of masked chafer larvae (Cyclocephala) but apply, in almost all details, to any larvae of this group. If you are interested in species identification, refer to the Web links in the bibliography. Green June beetle grubs have the interesting habit of crawling upside down, i.e. on their backs, over the ground surface and will do so if you place one on the ground or in a culture dish in the lab.
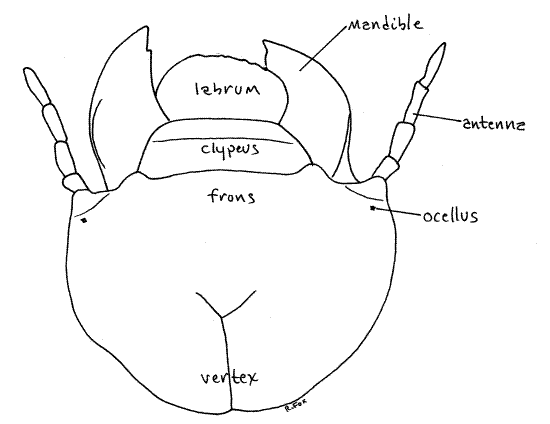
Figure 2. En face view of the head capsule of a Cyclocephala grub. Beetle72L.gif
Laboratory Specimens
Larvae can be collected from suburban lawns and gardens by digging in the sod and underlying soil. The following collecting technique minimizes damage to the sod. Cut through the sod on three sides of a 30-cm square and roll the turf away from the soil along the fourth, uncut, edge of the square. Remove the underlying, now exposed, 5 cm of soil and pass it through a 5-mm sieve to retain any grubs present. Replace the soil, move the sod back into position, and water it. In dry weather the sod should be watered a day in advance of collecting to attract the grubs from lower, moister levels of the soil.
Once collected grubs can be studied alive after anesthetization with chloroform or they can be preserved with 40% isopropanol or 80% ethanol. The exercise is confined to external anatomy and, although written specifically for Cyclocephala, can be used for the larvae of any scarab beetle.
External Anatomy
Scarab beetle grubs are white, with a brown head capsule and a brownish posterior end. They are about 25-40 mm in body length and 4-6 mm in diameter. The wormlike body is curved in a "C" shape (Fig 1). The body is divided into the usual three insect tagmata; head, thorax, and abdomen.
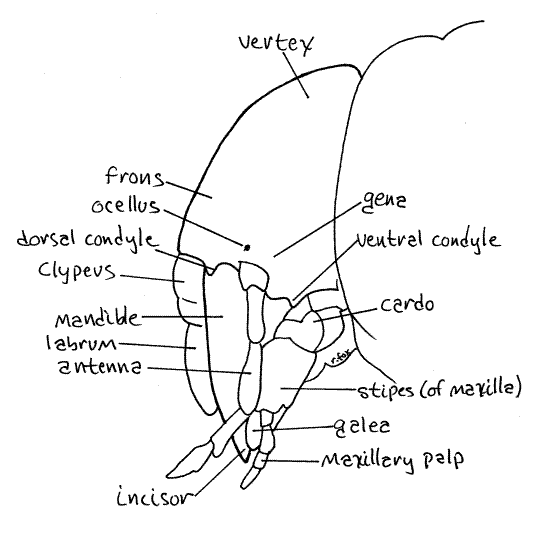
Figure 3. Left lateral view of the head of Cyclocephala. Beetle73L.gif
Head
The head consists of a dark, well-developed, sclerotized head capsule equipped with sense organs and mouthparts (Fig 2, 3). The vertex is the dorsal region of the capsule and it grades imperceptibly into the frons, the front of the capsule. The clypeus articulates with the ventral edge of the frons. The sides of the capsule are the genae, or cheeks (Fig 3).
Study the appendages of the head observing them first in anterior view and then in posterior view as required. The antenna arises from the ventral edge of the gena. No compound eyes are present but a tiny black ocellus is barely visible through the cuticle at the base of the antenna.
Of the mouthparts, only the labrum and mandibles are visible in anterior view. The oval, but asymmetrical, labrum articulates along a straight suture with the ventral edge of the clypeus (Fig 2, 3). The posterior surface of the labrum bears the epipharynx, prominent, median, posteriorly directed tooth that can be seen only in posterior view (Fig 4). The labrum is not bilobed.
The massive mandibles articulate laterally with the ventral edges of the genae (Fig 2, 3). The heavily sclerotized mandibles each have a distal incisor for shearing and a massive proximal molar, or molars, for grinding (Fig 4). Lift the labrum to reveal the full extent of the heavily sclerotized mandibles. The molars are best seen in posterior view after moving (or removing) the labium and maxillae.
Each mandible articulates with the gena via two contact points, or condyles. The dorsal and ventral condyles can be seen in lateral view at the border of the clypeus (Fig 3). A dicondylic articulation such as this limits movement to a single plane. The mandibles can move only in the transverse plane and thus oppose each other across the midline, incisor opposite incisor and molar opposite molar. Dicondylic articulations are typical of insect mandibles and several of the joints of insect legs. The human knee and elbow joints are also examples of dicondylic joints.
Figure 4. Posterior view of the mandibles and labrum. Maxilla and labium removed for clarity. Beetle74L.gif
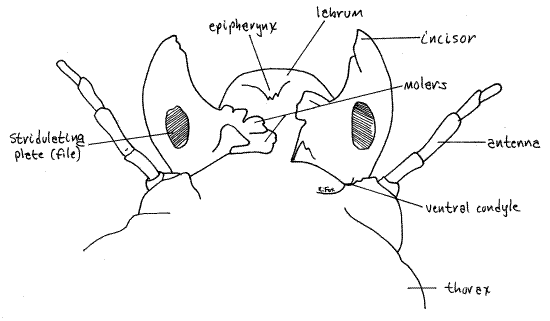
On the posterior face of the mandible, and visible only with the maxillae and labium out of the way or removed, is an oval patch, the stridulating plate, with fine striae (Fig 4). Higher magnification, about 40X or more, will be required to resolve the striae. The stridulating plate of the mandible is half of a stridulating device used by the grub to produce sound. The device consists of a file and scraper similar to those on the wings of male crickets (Acheta). The file is the stridulating plate of the mandible and the scraper is a row of bumps on the maxilla (Fig 6). The scraper lies against the file when the mandible and maxilla are in their natural positions. To stridulate, the grub rubs the scraper over the file. Also visible in posterior view of each mandible is an accessory condyle extending from the base of the mandible.
The maxilla is best viewed from the posterior side of the head, using fine forceps and a microneedle to manipulate it. The two maxillae are lateral to the labium so the three are simultaneously visible in posterior view (Fig 5). Each maxilla consists of a basal cardo articulated with the head capsule proximally and the stipes distally.
From the stipes arise a lateral maxillary palp and a fused galea and lacinia (Fig 5, 6). The presence of both galea and lacinia is apparent only in anterior view (Fig 6). In posterior view the two appear to be a single entity. The galea is lateral and the lacinia medial. The combined galea-lacinia bristles with stout spines and setae.
Figure 5. Posterior view of the maxillae and labium of Cyclocephala. Beetle75L.gif
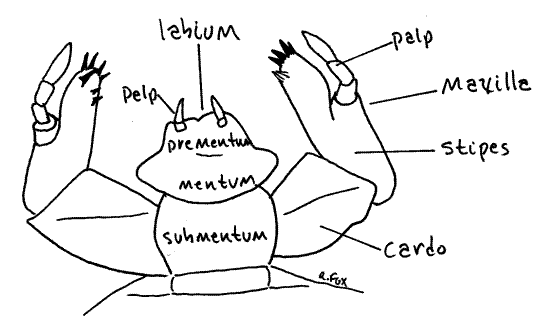
Figure 6. Anterior view of the labium and maxillae of Cyclocephala. Beetle76L.gif
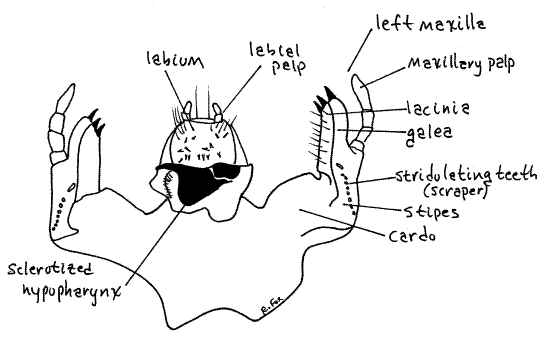
The anterior edge of the stipes bears a longitudinal ridge of sclerotized tubercles known as stridulating teeth (Fig 6). The stridulating teeth form the scraper that is the complement of the mandibular file of the stridulating organ (Fig 4).
Look at an intact labium in posterior view. It is median, between the two maxillae (Fig 5). It consists of a proximal submentum and a distal prementum with a mentum in between. Two tiny biarticulate labial palps arise distally from the mentum (Fig 3).
The hypopharynx, or tongue, in most insects is a soft, unsclerotized fold of the ventral wall of the head posterior to the mouth. In white grubs it is fused with the prementum of the labium and is heavily and asymmetrically sclerotized (Fig 6). Its black cuticular parts are clearly visible on the anterior base of the labium.
Figure 7 Left lateral view of thorax. Setae omitted for clarity. Beetle77L.gif
Thorax
As in the larvae or most holometabolous insects the posterior tagmata, thorax and abdomen, are weakly sclerotized making it difficult to distinguish segments. The body is soft and worm-like.
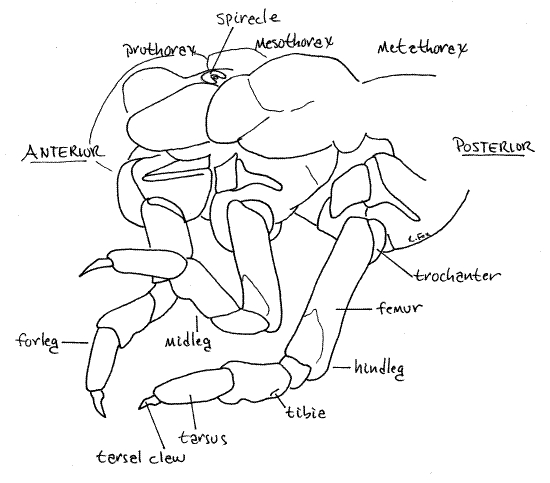
As always, the thorax consists of 3 segments which are the prothorax, mesothorax, and metathorax (Fig 1, 7). Each thoracic segment bears a pair of walking legs known, in order, as the forelegs, midlegs, and hindlegs (Fig 7). They are similar and each consists of coxa, trochanter, femur, tibia, tarsus, and single tarsal claw. A pair of spiracles is present laterally on the prothorax (Fig 1, 7).
Abdomen
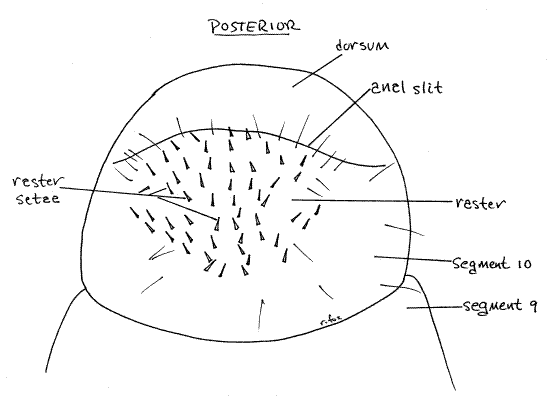
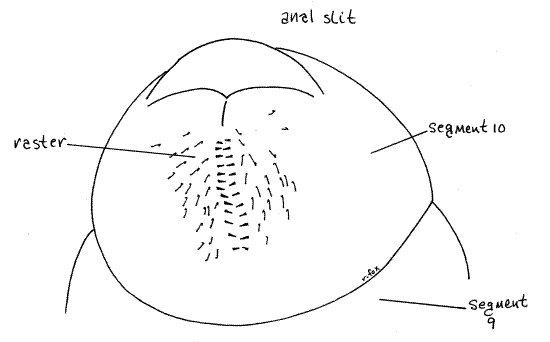
The abdomen consists of 10 segments but they are soft and mostly without distinct sclerites. Abdominal segments 1-8 each have a pair of spiracles (Fig 1) but spiracles are not present on segments 9 and 10. In Cyclocephala, but not Phyllophaga, segment 10 has a slender sclerotized tergite arching dorsally over the segment. The anus is a horizontal, transverse slit extending across the posterior end of segment 10. The region of segment 10 ventral to the anal slit is known as the raster and it bears a species-specific pattern of short spines (Fig 1, 8).
Figure 8. Ventral view of the posterior abdomen showing the random setal pattern characteristic of the raster of Cyclocephala. Setae have been omitted from the dorsum of segment 10. beetle78L.gif
Identification
Field identification of white grubs is accomplished by county extension agents, turf grass managers, and homeowners using the pattern of short setae or spines on the raster, or ventral surface of abdominal segment 10 (Fig 8). The pattern is discernable in the field using a 10X lens. In the two species of chafers (Cyclocephala) the pattern of spines is random (Fig 8). Some of the spines of Japanese beetles (Popillia), on the other hand, form a distinct "V" immediately anterior to the anal slit. May-June beetles (Phyllophaga) have two parallel, or slightly curved, closely spaced rows of spines in conjunction with a doubly arched anal slit (Fig 9). Green June beetles (Cotinus) have several long parallel rows of spines. Refer to the Internet sites below for illustrations of these, and other, raster patterns.
Figure 9. Ventral view of the posterior abdomen showing the random setal pattern characteristic of the raster of a May-June beetle, Phyllophaga.
References
Anon. undated. University of Kentucky Agripedia, Entomology 320 Horticultural Entomology.
www.ca.uky.edu/agripedia/classes/ENT320/TURFA2.asp
Böving AG, Craighead FC. 1930. An illustrated synopsis of the principal larval forms of the order Coleoptera. Entomologica Americana 11(ns)(1):1-244..
Böving AG, Craighead FC. 1931. An illustrated synopsis of the principal larval forms of the order Coleoptera. Entomologica Americana 11(ns)(4):257-352.
Borrer DJ, Triplehorn CA, Johnson NF. 1989. An introduction to the study of insects, 6 th ed. Saunders, Philadelphia. 875pp.
Hudson WH, Sparks B. undated. White grub pests on turfgrass. http://pubs.caes.uga.edu/caespubs/pubcd/1428-w.html
Ruppert EE, Fox RS, Barnes RB. 2004. Invertebrate Zoology, A functional evolutionary approach, 7 th ed. Brooks Cole Thomson, Belmont CA. 963 pp.
Russell H . 2001. Identifying white grubs in Michigan. www.pestid.msu.edu/profiles/whitegrubidentification.html With good photos and explanations of raster patterns for identifying white grubs.
Sheltar DJ . 2000. Identification of white grubs in turfgrass.
http://ohioline.osu.edu/hyg-fact/2000/2510.html
Supplies
living or preserved white grub (scarab beetle larva)
dissecting microscope
dissecting set with fine forceps and microneedles
small dissecting pan (anchovy tin)