Invertebrate Anatomy OnLine
Branchiostoma ©
Amphioxus
5jul2006
Copyright 2001 by
Richard Fox
Lander University
Preface
This is one of many exercises available from Invertebrate Anatomy OnLine , an Internet laboratory manual for courses in Invertebrate Zoology. Additional exercises, a glossary, and chapters on supplies and laboratory techniques are also available at this site. Terminology and phylogeny used in these exercises correspond to usage in the Invertebrate Zoology textbook by Ruppert, Fox, and Barnes (2004). Hyphenated figure callouts refer to figures in the textbook. Callouts that are not hyphenated refer to figures embedded in the exercise. The glossary includes terms from this textbook as well as the laboratory exercises.
Systematics
ChordataP, Metameria, Cephalochordata sP, Branchiostomatidae F (Fig 27-12, 29-32)
Chordata P
Chordata is characterized by a suite of apomorphies including a dorsal hollow nerve cord, notochord, pharyngeal gill slits, and a post anal tail (Fig 29-1). The ancestor was a fishlike deuterostome that swam using alternating contractions of right and left longitudinal axial muscles to create undulations of the body. The flexible, incompressible notochord prevented these contractions from compressing the body while allowing lateral deflection. The chordate central nervous system is a hollow, median, longitudinal nerve cord formed in the embryo by an invagination of surface ectoderm whose original function was probably sensory reception. Paired pharyngeal gill slits connect the lumen of the pharynx with the exterior and originally functioned in suspension feeding with respiration being added later. A muscular tail posterior to the anus is, although commonplace in chordates, an unusual feature not found in other taxa. It is an extension of the axial musculature and is the chief locomotory organ. An additional apomorphy is the endostyle, a region of pharyngeal endoderm, that secretes iodated compounds, either mucus or hormones.
Cephalochordata
Cephalochordates retain many of the features of the ancestral chordate including the dorsal hollow nerve cord, notochord, postanal tail, and pharyngeal gill slits used for filter feeding. The swimming and feeding modes are like those hypothesized for the ancestor.
Introduction
As the most vertebrate-like of invertebrates, cephalochordates are studied in either vertebrate or invertebrate zoology courses. Comparative vertebrate anatomy traditionally begins with a consideration of cephalochordates and other protochordates and invertebrate zoology often ends with them. Protochordates are the invertebrate members of Chordata and include urochordates (sea squirts), and cephalochordates (amphioxus, lancelets). These taxa are clearly related to vertebrates but also show unmistakable relationships with other invertebrates, most notably echinoderms and hemichordates. These then are the taxa that establish the evolutionary connections between the vertebrates and rest of the deuterostomes and ultimately, the remainder of the animal kingdom. Cephalochordates probably diverged from the evolutionary line leading to the vertebrates before the end of the Precambrian.
Of the protochordate taxa, it is Cephalochordata that is the most vertebrate-like (Fig 29-32). These fishlike marine animals have the expected chordate characteristics of dorsal hollow nerve cord, pharyngeal gill slits, notochord, and post-anal tail. Although they lack the brain, primary sense organs and sense capsules, braincase, bones, kidneys, heart, an endothelial lining of the blood vessels, and vertebrae, which characterize most vertebrates, they share many other features with vertebrates. Cephalochordates are laterally compressed and fishlike in general morphology, have segmentally arranged axial (body) muscles, and a vertebrate-like hemal system. As you study these animals, watch for other features that remind you of vertebrates.
Cephalochordata is a small taxon of 30 species in only two genera, Branchiostoma and Epigonichthyes (= Asymmetron ). Cephalochordates are generally known by the common name "lancelet" and those used in laboratories are usually referred to as "amphioxus", from a now obsolete generic name. Amphioxus means “sharp at both ends, an apt name for these animals as you will soon see. All species are less than 10 cm in length and most are closer to five or less. They are segmented, coelomate deuterostomes.
Lancelets inhabit shallow offshore sands in temperate oceans with only the head protruding above the sand surface (Fig 29-4). They are capable of swimming briefly using lateral undulations of the body caused by contractions of the axial musculature. They are efficient burrowers, entering the sand head first, employing side-to side undulations of the body similar to those used in swimming. Lancelets are harvested for human consumption in some parts of the Orient.
Cephalochordates are filter feeders that use cilia to generate a current of water and phytoplankton particles into the mouth (Fig 29-6). Water and food enter the pharynx where they are separated by filtration. The water, but not the food, passes out through the gill slits and thus leaves the gut cavity. It then enters a surrounding water space, the atrium, and moves from there to the exterior. Most vertebrates have no structure like the atrium so it may be difficult to visualize this relationship at first. Urochordates, on the other hand, have exactly the same filter feeding apparatus (Fig 29-23). The food particles are too large to pass through the gill slits and must remain in the lumen of the pharynx from which cilia move them posteriorly into digestive and absorptive regions of the gut. The original function of the perforated pharynx (gill slits) of the chordates is thought to have been filter feeding with gas exchange being added much later in chordate evolution. More complete discussions of the biology of cephalochordates may be found in Ruppert (1997), Ruppert, Fox, and Barnes (2004), Parker and Haswell (1921), Nash (2002), and Jollie (1973).
Anatomy
1. Amphioxus: Adult specimen (Fig 1, 2). Begin your study with a preserved adult animal in a small dish of tapwater. Handle the specimen carefully as the preservative renders them brittle. Place the dish on the stage of your dissecting microscope and use a teasing needle to manipulate it. You will study only the external anatomy of this specimen and will not dissect it. Later you will study the internal anatomy using wholemounts and cross sections.
The integument (= skin) is the outer covering (Fig 29-3D). It includes a monolayered, nonciliated epidermis with a basal lamina. There is no secreted extracellular cuticle. Below the epidermis is a thick, collagenous, translucent, gelatinous, connective tissue dermis. In life the entire animal is translucent but preserved specimens are opaque.
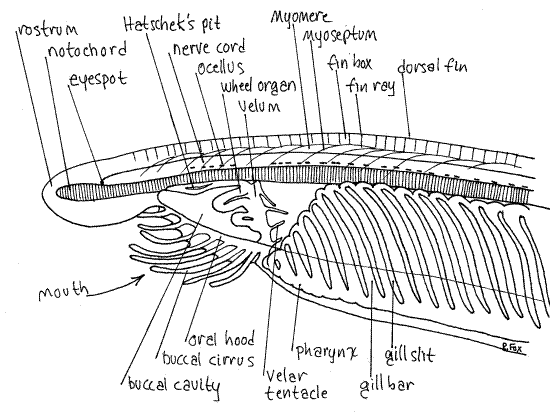
Note the fishlike (fusiform or lanceolate) shape of the animal (Fig. 1, 29-3A). The body is divided into head, trunk, and tail. The head, at the anterior end, is small and poorly defined. The rostrum extends anteriorly and overhangs the mouth and buccal cavity (Fig 2, 29-5). The large mouth lies under the rostrum and opens into a spacious buccal cavity. The mouth is surrounded by a ring of tentacle-like buccal cirri (=oral cirri). These are involved in preliminary mechanical sorting of food particles and are probably chemoreceptive as well. The roof and walls of the buccal cavity form the oral hood.
Most of the body is the trunk (Fig 1, 29-3B), which extends posteriorly from the head to the anus (the anus will be easier to see later when you study a cleared wholemount). The trunk contains most of the gut, including the large conspicuous pharynx (not visible at present), and the musculature. The segmental arrangement of the axial musculature (body musculature) is readily apparent through the translucent integument. The muscles are arranged in 50-75 V-shaped segmental bundles called myomeres. Successive myomeres are separated from each other by connective tissue partitions called myosepta. The segmental organization of animals is referred to as metamerism and the vertebrates, as well as several other phyla of animals, such as annelids and arthropods, are said to be metameric. Myomeres on opposite sides of the body are asymmetric and out of register with each other (Fig 3).
There are no paired appendages but on either side of the trunk is a ventro-lateral longitudinal ridge, the metapleural fold (Fig. 1, 3). These ridges run from the oral hood to a position just posterior to the gonadal region. The atrium, which is not visible in whole specimens, opens to the exterior via the atriopore, located on the midventral margin at the point where the two metapleural folds join the ventral margin. Farther posteriorly, beside a slight dip between the ventral fin and the caudal fin, is the anus, located slightly to the left side of the ventral midline. The anus is the posterior external opening of the gut and marks the posterior limit of the trunk. The region of the body posterior to the anus is the tail. One of the characteristics of chordates is the presence of this postanal tail.
A posterior caudal fin (=tail fin) extends around the dorsal and ventral margins of the tail. There is a long dorsal fin along most of the dorsal margin of the body. A short ventral fin is located on the ventral margin of the trunk just anterior to the caudal fin. It extends from atriopore to anus.
Figure 1. Juvenile Branchiostoma, cleared specimen. Cephchord16L.gif
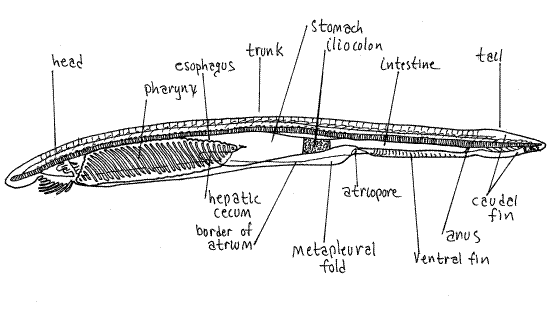
Segmentally arranged, paired, rectangular swellings along the ventral margins of the myomeres are about 26 pairs of segmental gonads (Fig 29-3A,B). Branchiostoma is gonochoric. The gametes are shed into the atrium and are carried to the exterior by the water passing through it and out the atriopore.
2. Amphioxus: wholemount of juvenile specimen (Fig 1, 2). Continue your study of with a wholemount of a cleared juvenile amphioxus. These specimens are much smaller than the mature specimen and are sexually immature. Mature individuals are too large and thick for examination with a compound microscope. Juveniles are identical to adults except for their size and lack of gonads. Use of a small cleared specimen permits examination of many features of the internal anatomy without dissection. More detailed structures and relationships will be studied at higher power using cross sections.
Place the slide on the stage of the compound microscope so that the animal is upside down when viewed with the unaided eye. It will, of course, be right side up when observed through the microscope. Study the animal using 40X, and occasionally 100X, as appropriate. You will see many features familiar to you from your study of the adult specimen. Relocate and re-identify the rostrum, head, dorsal fin, caudal fin, ventral fin, tail, oral hood, buccal cirri, metapleural folds, and atriopore.
Look at the dorsal region of the animal and find the dorsal fin, recognizable by its faint vertical fin rays. The fin is composed of a longitudinal series of coelomic spaces each known as a fin box (Fig 2, 29-5B). The fin rays are the septa between successive fin boxes. Follow the dorsal fin anteriorly and posteriorly noting that it extends along most of the dorsal midline.
Figure 2. Anterior end of a cleared juvenile Branchiostoma. Cephchord17L.gif
Using 100X and careful focusing, you should be able to see the myomeres that make up most of the body. The myosepta appear as faint, oblique, V-shaped lines. The myomeres and myosepta extend ventrally to the metapleural folds but they are hard to see against the confusing background in this region. Myomeres are derived from segmental coelomic compartments and the muscles are derived from the epitheliomuscular cells of the coelomic mesothelium (peritoneum).
The dorsal hollow nerve cord extends most of the length of the body (Fig 1, 2). It is usually pinkish in these preparations and has a distinctive, irregular, longitudinal row of black, light-sensitive, pigment cup ocelli running along its ventral margin. These ocelli face in various directions, some dorsal and some ventral. The nerve cord extends anteriorly to about the base of the rostrum where it bears a larger, terminal ocellus known as the eyespot. The nerve cord extends posteriorly into the base of the tail.
Although you will not see them, the nerve cord gives rise to paired segmental dorsal and ventral nerves, homologous to the spinal nerves of the vertebrates. Paired sensory and visceral motor nerves connect the nerve cord but somatic motor neurons are absent, or nearly so. Instead, the axial muscles and the cells of the notochord are innervated by cytoplasmic processes of the muscle cells extending to the nerve cord. The nerve cord is surrounded by a connective tissue sheath.
The cavity of the hollow nerve cord is the neurocoel. Anteriorly, the neurocoel opens to the exterior by a permanent, dorsal neuropore at the base of the rostrum (Fig 29-5B). Chemoreceptors in the neuropore monitor the water in its vicinity. The lumen of the nerve cord (neurocoel) is expanded anteriorly to form a vesicle sometimes referred to as the brain.
Ventral to the nerve cord is the notochord. It is longer, relative to the length of the body, in these animals than in any other chordate. It is longer than the nerve cord and extends well into the rostrum, presumably as an adaptation to facilitate digging into sand. The appellation "Cephalochordata" for these animals alludes to the presence of the notochord in the head. In vertebrates the notochord extends anteriorly only as far as the middle of the brain (mesencephalon). The notochord is usually yellowish in these preparations and appears to be vertically striated. It is composed of large, vacuolated, disklike epitheliomuscular cells arranged in a stiff longitudinal column and surrounded by a thick connective tissue sheath (Fig 29-4A,E).
The notochord resists the deformation of the body that would otherwise result from contraction of axial muscles. Embryologically, the notochord arises from a dorsal median thickening of the roof of the archenteron. The coelom of enterocoelic animals arises from paired dorso-lateral outpocketings of the archenteron roof.
The remainder of the animal is mostly digestive system. Find once more the buccal cirri surrounding the oral hood (Fig 29-5). The buccal cavity is the gut lumen within the oral hood. The posterior wall of the buccal cavity is the transverse, muscular velum. An aperture, of adjustable diameter, in the center of the velum connects the buccal cavity with the pharynx. Anterior to the velum, the walls of the buccal cavity bear a series of thick ciliated grooves which make up the wheel organ. The cilia in these grooves trap food particles in mucus for digestion farther posterior in the gut. On the posterior side of the velum, the mouth is surrounded by slender sensory velar tentacles.
On the dorsal midline of the buccal cavity is a deep ciliated fossa called Hatschek's pit or Hatschek’s nephridium (Fig 29-5B). This is an unpaired kidney whose duct opens into the anterior pharynx. It is difficult to avoid attempting to homologize this structure with Rathke`s pouch of vertebrate embryos and thus with the anterior pituitary of adult vertebrates. Like those structures, it is in the ectoderm of what amounts to the stomodeal region and it extends dorsally toward the anterior end of the nerve cord. Definite homology with the pituitary has not been demonstrated but structural and positional criteria support the supposition. Hatschek's pit is also secretory and releases mucus to entrap food particles.
The pharynx, as in most protochordates, is by far the largest, most conspicuous, and most distinctive region of the gut (Fig 29-6, 29-3C). It begins just posterior to the velum and is a large, intensely red-staining, oval structure with numerous, narrow, oblique gill slits separated by narrow tissue gill bars (Fig 29-5B). The gill bars are supported by a collagenous branchial skeleton presumably homologous to the visceral (gill) skeleton of vertebrates. The gills function primarily in filter feeding with the responsibility for gas exchange being met by the thin epidermis of the general body surface. It is worthy of note that in larvae the number of gill slits equals the number of myomeres. This is consistent with the hypothesis that that the gill slits, like the myomeres, were segmental structures in the ancestors of vertebrates.
The pharynx is surrounded by a large water chamber, the atrium, which is an invagination of the surface ectoderm (Fig 1, 3, 29-3C). The atrium occupies most of the space between the pharynx and the body wall. It is U-shaped in cross section and encloses the pharynx on all sides except dorsally. This spatial relationship will become clearer when you study the cross section slide. Although the atrium may seem to be an unusual adaptation entirely absent in the vertebrates, it is actually identical to the opercular cavity of larval anurans (tadpoles) and is similar to the opercular cavity of the bony fishes, both being water chambers outside the pharynx. The atrium opens to the exterior through a large atriopore (Fig 29-6).
Posterior to the pharynx the gut narrows to form a short esophagus which connects the pharynx with thestomach (midgut ). Anteriorly the stomach bears a ventral anteriorly-directed diverticulum, the secretory and absorptive hepatic cecum, which projects into the atrium on the right side of the pharynx (Fig 29-6). Students with experience in vertebrate embryology will remember that the vertebrate liver develops from a midgut diverticulum on the right side of the embryo. By the structural, positional, and intermediate criteria the hepatic cecum of amphioxus seems to be homologous to the liver of vertebrates.
The stomach narrows posteriorly to become the darker iliocolon. This region of the gut tube bears a ring of ciliated epithelium that rotates the food mass and mixes it with enzymes. Posterior to the ring the gut continues as the intestine, ultimately ending at the anus, located at the base of the tail. The asymmetrically located anus lies just to the left of the ventral fin. Thepostanal tail extends posteriorly from the anus.
3. Amphioxus: pharyngeal cross section (Fig 3, 29-3C). With low power of the compound microscope examine a slide of amphioxus cross sections and find the section through the pharyngeal region. Slides labeled "representative cross sections" will bear three or four sections taken through different regions of the body. Make sure you have the correct section (the one through the pharynx) by comparing it with Fig. 3. Use 40X, 100X, and occasionally 400X, for this exercise.
Note first the outer integument completely surrounding the animal. Although you cannot discern its composition, it consists of an outer acellular cuticle which is secreted by the underlying epidermis. Below the epidermis is the soft, connective tissue dermis. The integument bulges outward along the dorsal midline where it covers the dorsal fin.
Find the numerous myomeres along each side of the body and note that they are not bilaterally symmetrical (Fig 3). Each myomere is surrounded by a myoseptum composed of connective tissue. Dorsally between the right and left muscle masses is a relatively small nerve cord and a much larger notochord. Both are surrounded by sheaths of connective tissue.
Figure 3. Cross section of an adult Branchiostoma through the pharyngeal region. Cephchord18L.gif
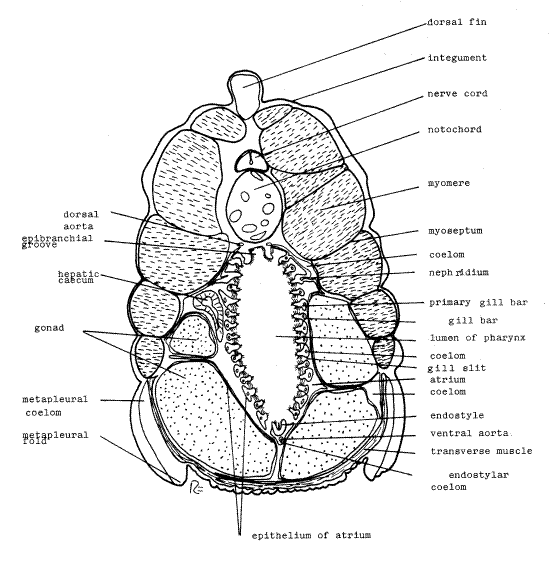
The interior of the ventral half of the section is occupied by the pharynx. Its lumen is surrounded by the gill bars, appearing here in oblique section. In living material the gill bars are nearly vertical but in most fixed specimens the pharynx is distorted so that the bars are aligned obliquely or nearly horizontally. As a consequence you see them in oblique or cross section rather than sliced along their long axis as you might have expected. Look closely at the circle of gill bars noting first the gill slits that separate them. It is through these slits that water passes during feeding. Examine some of the gill bars with 400X (careful!) and note the abundance of cilia on their inner margins. Lateral cilia (Fig 3, 29-3C) are located on the surfaces between the gill bars and generate the feeding current which passes from the pharynx lumen through the gill slits to the atrium. In contrast frontal cilia are on the inner surface of the gill bars face the lumen and are responsible for transporting food and mucus dorsally to the esophagus. Each gill bar is associated with a small coelomic space and an aortic arch but these are difficult to see.
Ventrally along the pharynx there is a longitudinal, median, ciliated groove, the endostyle, which secretes a copious mucus. The mucus contains iodine and the endostyle is homologous to the vertebrate thyroid gland (positional and intermediates criteria). Look at the endostyle with high power to find its cilia. Ventral to the endostyle there is a small endostylar coelom surrounding an even smaller ventral aorta. The endostylar coelom is homologous to the pericardial coelom of vertebrates.
Dorsally in the pharynx there is another median furrow, the epibranchial groove. The paired dorsal aortae are located dorsolateral to it. Most of the space surrounding the pharynx is the atrium, which is enclosed by an ectodermal epithelial lining.
During feeding the lateral cilia of the gill bars generate a water current that enters the pharynx through the mouth. The water passes laterally through the gill slits, out of the pharynx, and into the surrounding atrium. Mucus secreted by the endostyle is carried upward over the inner surface of the gill bars by ciliary currents generated by frontal cilia. The mucus entangles food particles attempting to pass between the gill bars and transports them to the epibranchial groove. The cilia of the groove then move the mucus and trapped food posteriorly into the esophagus. Food is moved through the gut by its ciliated walls. The epithelium of the hepatic cecum secretes digestive enzymes into the gut lumen. Digestion occurs extracellularly in the stomach and iliocolon and its products are absorbed by the epithelium of the cecum.
Although inconspicuous, the cephalochordate coelom occupies its expected position surrounding the gut (Fig 29-3C, 29-9). Fairly large perivisceral coelomic spaces may be seen lying dorsolateral to the pharynx. Extensions of this part of the coelom extend to the gill bars and are continuous with the coelom in those structures.
Paired segmental nephridia, which you may not see, are located in the perivisceral coelomic spaces beside the pharynx (Fig 29-9). These unusual nephridia have podocytes like metanephridia but also have a flagellated collar cell and weir basket like protonephridia. The podocytes are associated with the dorsal aorta and form an ultrafiltrate of the blood into the perivisceral coelom. To leave the coelom and enter the atrium for elimination, the urine must pass through the weir basket of a flagellated collar cell.
The ventrolateral regions of mature specimens are occupied by large gonads, either ovary or testis (Fig 29-3A,B). Determine the sex of your specimen by examining the contents of its gonads. Eggs will be large and easy to see, sperm very small and unrecognizable. What is the sex of your specimen? ___________. You are responsible for recognizing gonads of both sexes. The gonads are enclosed in narrow coelomic spaces which may be difficult to see.
Note the transverse muscle ventral to the gonads. The metapleural folds are folds of the body wall and they contain a coelomic space, the pterygocoel (=metapleural coelom, Fig 3, 29-3C). The hepatic cecum, enclosed in a small coelomic space, may be seen in cross section beside the pharynx. Posterior to the pharyngeal region the coelom is larger and surrounds the intestine.
The pattern of blood flow in amphioxus is similar to that of early vertebrates although there is no distinct heart (Fig 29-8). The ventral aorta in the pharyngeal region gives rise to numerous pairs of aortic arches that pass dorsally through the gill bars. Upon leaving the gills the arches unite to form a pair of longitudinal dorsal aortae dorsal to the pharynx. The dorsal aorta carries blood posteriorly to muscles and gut. Blood is returned to a ventral sinus venosus under the pharynx by a system of anterior and posterior cardinal veins (which are not evident on these slides). The sinus venosus is formed by the junction of the cardinal veins. The ventral aorta extends anteriorly from the sinus venosus.
There is also a visceral venous drainage system associated with the gut and hepatic cecum that is probably homologous to the hepatic portal system of vertebrates. Blood leaves capillaries in the gut wall via the hepatic portal vein that carries it to capillary beds in the cecum. From there the hepatic vein transports it to the sinus venosus. An hepatic portal system is a universally present feature of vertebrates.
The ventral aorta is contractile in amphioxus and functions as the heart but there are also tiny pulsatile vesicles in the aortic arches. The blood is acellular and unpigmented and probably plays no significant role in gas transport. Hemoglobin is, however, present in muscles and notochord (which is muscular). The vessels lack an endothelial lining (like other invertebrates but unlike vertebrates).
Bibliography
Jollie M. 1973. Chordate morphology. Krieger, Huntington, NJ. 478 pp.
Nash TR. 2002. Feeding biology, digestive physiology, and metabolic rate of the Florida lancelet, Branchiostoma floridae. PhD. Dissertation, Zoology, Clemson University, Clemson, SC. 174 pp.
Parker TJ, Haswell WA. 1921. A textbook of zoology, vol 2, 3rd ed. MacMillan, London. 714 pp.
R ä hr H. 1979. The circulatory system of amphioxus (Branchiostoma lanceolatum PallasP. Acta Zool. 60:1-18.
Ruppert EE. 1997. Cephalochordata (Acrania), In Harrison FW, Ruppert EE (Eds): Microscopic anatomy of Invertebrates, vol 15. Wiley-Liss, New York. Pp 349-504.
Ruppert EE, Fox RS, Barnes RB . 2004. Invertebrate Zoology, A functional evolutionary approach, 7 th ed. Brooks Cole Thomson, Belmont, CA. 963 pp.
Stokes MD, Holland ND. 1998. The lancelet. American Sci. 86(6):552-560.
Supplies
Syracuse Dish
dissecting microscope
compound microscope
1 preserved adult amphioxus
1 wholemount slide, juvenile amphioxus
1 cross section slide including the pharyngeal region