Invertebrate Anatomy OnLine
Artemia franciscana
Brine Shrimp ©
19jun2006
Copyright 2001 by
Richard Fox
Lander University
Preface
This is one of many exercises available from Invertebrate Anatomy OnLine , an Internet laboratory manual for courses in Invertebrate Zoology. Additional exercises can be accessed by clicking on the links to the left. A glossary and chapters on supplies and laboratory techniques are also available. Terminology and phylogeny used in these exercises correspond to usage in the Invertebrate Zoology textbook by Ruppert, Fox, and Barnes (2004). Hyphenated figure callouts refer to figures in the textbook. Callouts that are not hyphenated refer to figures embedded in the exercise. The glossary includes terms from this textbook as well as the laboratory exercises.
Systematics
Arthropoda P, Mandibulata, Crustacea sP, Eucrustacea, Thoracopoda, Phyllopodomorpha, Anostraca C, Artemiidae F (Fig 16-15, 19-90)
Arthropoda P
Arthropoda, by far the largest and most diverse animal taxon, includes chelicerates, insects, myriapods, and crustaceans as well as many extinct taxa such as Trilobitomorpha. The segmented body primitively bears a pair of jointed appendages on each segment. The epidermis secretes a complex cuticular exoskeleton which must be molted to permit increase in size. Extant arthropods exhibit regional specialization in the structure and function of segments and appendages but the ancestor probably had similar appendages on all segments. The body is typically divided into a head and trunk, of which the trunk is often further divided into thorax and abdomen.
The gut consists of foregut, midgut, and hindgut and extends the length of the body from anterior mouth to posterior anus. Foregut and hindgut are epidermal invaginations, being derived from the embryonic stomodeum and proctodeum respectively, and are lined by cuticle, as are all epidermal surfaces of arthropods. The midgut is endodermal and is responsible for most enzyme secretion, hydrolysis, and absorption.
The coelom is reduced to small spaces associated with the gonads and kidney. The functional body cavity is a spacious hemocoel divided by a horizontal diaphragm into a dorsal pericardial sinus and a much larger perivisceral sinus. Sometimes there is a small ventral perineural sinus surrounding the ventral nerve cord.
The hemal system includes a dorsal, contractile, tubular, ostiate heart that pumps blood to the hemocoel. Excretory organs vary with taxon and include Malpighian tubules, saccate nephridia, and nephrocytes. Respiratory organs also vary with taxon and include many types of gills, book lungs, and tracheae.
The nervous system consists of a dorsal, anterior brain of two or three pairs of ganglia, circumenteric connectives, and a paired ventral nerve cord with segmental ganglia and segmental peripheral nerves. Various degrees of condensation and cephalization are found in different taxa.
Development is derived with centrolecithal eggs and superficial cleavage. There is frequently a larva although development is direct in many. Juveniles pass through a series of instars separated by molts until reaching the adult size and reproductive condition. At this time molting and growth may cease or continue, depending on taxon.
Mandibulata
Mandibulata is the sister taxon of Chelicerata and in contrast has antennae on the first head segment, mandibles on the third, and maxillae on the fourth. The brain is a syncerebrum with three pairs of ganglia rather than the two of chelicerates. The ancestral mandibulate probably had biramous appendages and a J-shaped gut, posterior-facing mouth, and a ventral food groove. The two highest level mandibulate taxa are Crustacea and Tracheata.
Crustacea sP
Crustacea is the sister taxon of Tracheata and is different in having antennae on the second head segment resulting in a total of 2 pairs, which is unique. The original crustacean appendages were biramous but uniramous limbs are common in derived taxa. The original tagmata were head but this has been replaced by head, thorax, and abdomen or cephalothorax and abdomen in many taxa. Excretion is via one, sometimes two, pairs of saccate nephridia and respiration is accomplished by a wide variety of gills, sometimes by the body surface. The nauplius is the earliest hatching stage and the naupliar eye consists of three or four median ocelli.
Eucrustacea
Eucrustacea includes all Recent crustaceans except the remipedes. The taxon is characterized by a primary tagmosis consisting of heat, thorax, and abdomen although the derived condition of cephalothorax and abdomen is more common. Eight is the maximum number of thoracic segments.
Thoracopoda
In the ancestral thoracopod the thoracic appendages were turgor appendages used for suspension feeding in conjunction with a ventral food groove. Such appendages and feeding persist in several Recent taxa but have been modified in many others.
Phyllopodomorpha
The compound eyes are stalked primitively although derived sessile eyes occur in many taxa.
Anostraca C
Anostraca is an ancient line of primitive crustaceans found today in inland, relictual, waters such as snow-melt pools, wet weather ponds, saline or alkaline lakes, or similar refugia where there are few or no predators.
Anostracans have no carapace (anostraca = without shell) and are thought to be similar in many features to the ancestral crustaceans. The body is elongate and shrimp-like and with little regional specialization of segments or appendages. The heart is a long dorsal tube extending most of the length of the body and equipped with segmental ostia. Anostracans are gonochoric and the gonads are paired, tubular coelomic derivatives. The excretory organs are saccate nephridia derived from coelomic remnants.
The gut is J-shaped with paired digestive ceca. Most anostracans are suspension feeders and/or bottom scrapers. The thoracic appendages are broad, flat phyllopods resembling those of the ancestral crustacean. The exoskeleton is thin, flexible, only weakly sclerotized and is not calcified. The ladder-like nervous system is typical of primitive arthropods (and annelids) and exhibits no cephalization of ventral ganglia. Development includes nauplius and zoea larvae.
Laboratory Specimens
Anostracans are the best examples of primitive crustacean morphology readily available for study in introductory invertebrate zoology courses. Of the crustaceans usually available for study in teaching laboratories, anostracans are the most primitive and provide the closest approximation to the presumed condition of the ancestral crustaceans.
Brine shrimp, in the genus Artemia, inhabit inland saline lakes worldwide. They are tolerant of a wide range of salinities ranging from almost fresh water to saturated. Artemia is easy to rear from inexpensive, commercially available eggs and is thus easily studied alive at any time of the year. Artemia franciscana is the most common North American species. It occurs in Great Salt Lake and is the species whose eggs are sold commercially in North America. This species produces desiccation-tolerant resting eggs that remain viable in dry condition for several years. These eggs can be purchased inexpensively from Carolina Biological, Ward's Natural Science, or at local pet stores. With little effort the eggs can be hatched to produce living nauplius, advanced larvae, and adults for the laboratory. Rearing instructions are provided at the end of this exercise. Artemia is restricted to saline lakes and consequently cannot be collected locally in most places.
Anostraca is a homogeneous taxon and its members are similar to each other so that any species can be used for this study. If possible, living specimens should be used but preserved specimens will also serve. Preserved specimens are suitable for the study of external anatomy but are much inferior for observation of internal structures. Fairy shrimps, such asEubranchipus, occur in temporary ponds and ditches, are morphologically similar to Artemia, and can be used for this study. Fairy shrimps are widespread but their occurrence is spotty and unpredictable and they are not available alive from most supply houses. If they are available from local wet weather ponds they are recommended for this exercise. Commercially prepared wholemount slides of Eubranchipus are less satisfactory than unmounted preserved specimens.
External Anatomy
Use the dissecting microscope to study an adult brine shrimp (Artemia) or fairy shrimp (e.g. Eubranchipus). If the animal is alive, place it in a small square watch glass of chloroform-saturated water (See Supplies and Recipes Chapter). If the specimen is preserved, place it in a dish of tapwater. Use your minuten nadeln to manipulate the specimen and study it with the dissecting microscope.
Tagmata
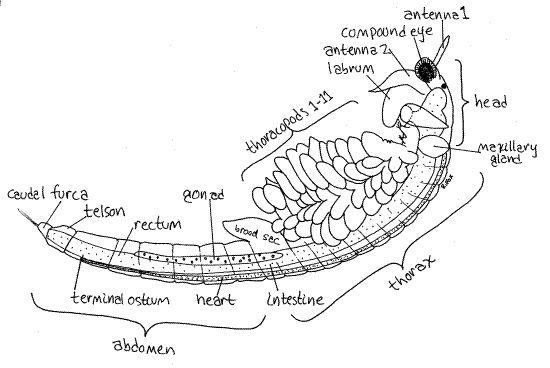
The anostracan body is divided into three tagma; head, thorax, and abdomen (Fig 1). The head and its appendages are specialized, as they were in the ancestral crustacean. The thoracic appendages are similar to each other and are unspecialized phyllopods. The abdomen lacks appendages.
Head
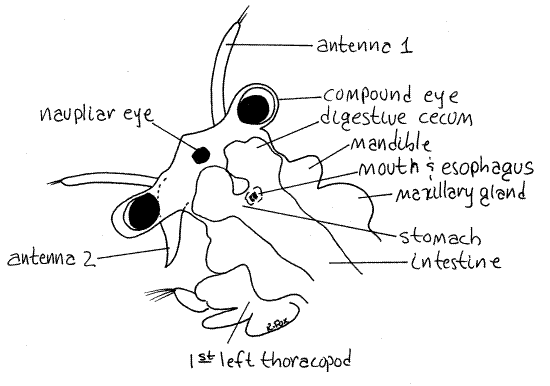
The head at the anterior end is composed of five coalesced segments as it is in all crustaceans (Fig 1). It bears a pair of stalked, lateral, compound eyes and a single, median, unstalked naupliar eye at the anterior end (Fig 2, 3). The eyes, although stalked, are not considered to be segmental appendages.
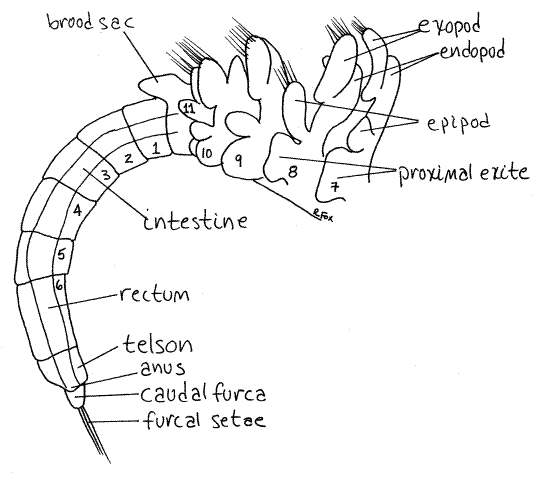
Figure 1. A female brine shrimp, Artemia franciscana, viewed from the left. Thoracopod setae omitted for clarity. Anostraca1La.gif
The small chemosensory first antennae are the appendages of the first head segment (Fig 1, 2, 3, 19-11A). They are uniramous, and unjointed.
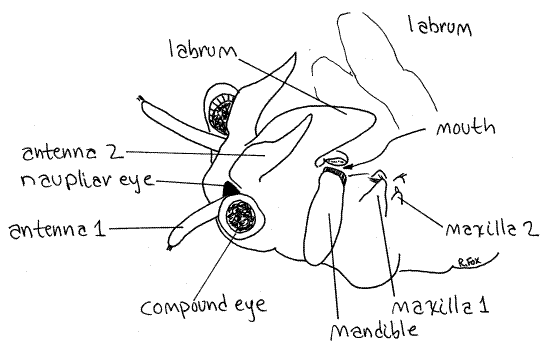
The second antennae are larger and are sexually dimorphic. Those of adult males are very large and modified to form a clasping organ to hold the female during copulation (Fig 19-11A). They are composed of two articles. Female second antennae are smaller, about the length of the first antennae but much thicker, and are composed of a single article (Fig 2). Determine the sex of your specimen.
The labrum, or upper lip, is a large, median, ventral fold of body wall arising just posterior to the bases of the second antennae (Figs 1, 2). It is not paired and is not a segmental appendage. It extends posteriorly and covers the ventral surface of the head, including the mouth.
The two, oval, bulging mandibles lie on either side of the head and are the appendages of the third head segment (Figs 1, 2, 3). The mandibles curve medially and touch each other on the midline where the ventral borders bear teeth.
The mouth is on the ventral midline between the two mandibles (Fig 2). It may be necessary to move the labrum aside to see the ventral ends of the mandibles and the mouth.
The first and second maxillae are small and difficult to see. The first maxilla is larger than the second and bears a bundle of anteriorly directed setae on its medial edge (Fig 2, 3). The first maxillae are immediately posterior to the mandibles on the ventral surface of the head and are used to transfer food from the thoracic appendages to the mouth. The tiny, conical second maxillae are vestigial and bear a few setae and the nephridiopores (Fig 2, 3).
The adult excretory organs are the two maxillary glands, or coxal glands, in the segment of the second maxillae where they form conspicuous bulges on its dorsolateral surfaces (Fig 3). These are typical crustacean saccate nephridia. Their coiled ducts may be visible within the bulges. The maxillary glands open via the nephridiopores on the second maxillae.
Figure 2. Oblique view of the right side of the head of a female Artemia. Anostraca2L.gif
Thorax
The remainder of the body is the segmented trunk consisting of an anterior, limb-bearingthorax and limbless, posterior abdomen. The thorax consists of 11 independent segments. No carapace is present and, since none of the thoracic segments is fused with either the head or with each other, there is no cephalothorax.
Each thoracic segment bears a ventral pair of leaflike thoracopods (= thoracic appendages) known asphyllopods (Figs 1, 3). The 11 pairs of phyllopods are similar to each other and exhibit no regional specialization, differing only in size. The phyllopods are turgor appendages in which the exoskeleton is thin and flexible and blood pressure is required to keep the appendages stiff. The phyllopods are used for swimming, feeding, and respiration.
The gross features of the phyllopod are visible with the dissecting microscope but observation of detail requires preparation of a wet mount and examination with the compound microscope. At present you should use the dissecting microscope to study intact phyllopods while they are attached to the animal (Fig 3). Later you will remove a phyllopod and make a wetmount. Do not remove a phyllopod until you have completed the study of the internal anatomy and then return to the following description for a detailed study. For now, find only the larger features visible with the dissecting microscope.
There are similarities between the parts of the phyllopods and the ancestral biramous appendage but proposed homologies between these parts are not universally accepted. The appendages are functionally uniramous although they have parts that are thought to be homologous to the two rami of a biramous appendage. Each appendage is flat and leaflike (phyll = leaf) and thus resembles the phyllopodous portion of the ancestral biramous mixopod. There is no stenopodous (cylindrical) portion.
Figure 3. Lateral view of the left side of the head and anterior thorax of a female Artemia. Anostraca3L.gif
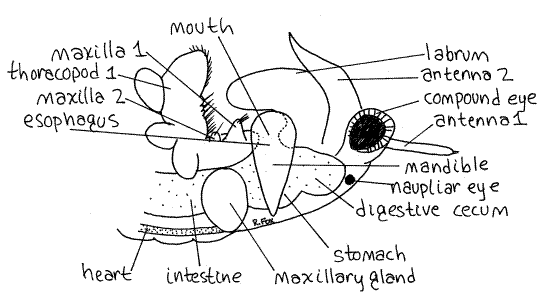
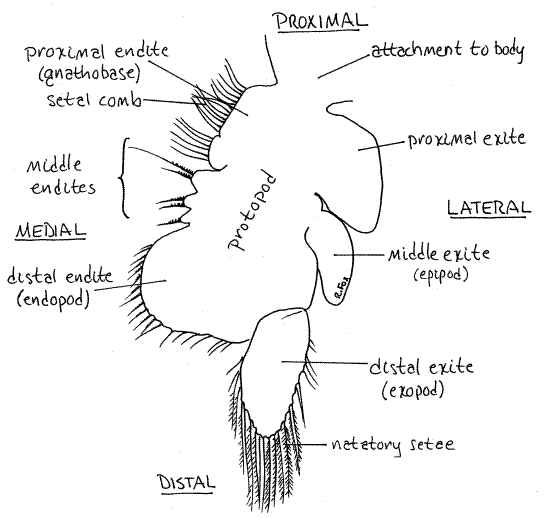
Find the large basal protopod attached along its dorsal edge to the body (Fig 4, 19-11B). Several processes extend from the lateral and medial borders of the protopod. Any process from the lateral border of a crustacean limb is an exite and any process from the medial border is an endite. You can see some of these with the dissecting microscope but they will be clearer later under the compound microscope.
Five or six endites, some of which are very small, extend from the medial margin of the protopod (Fig 4, 19-11B). The proximal and distal endites are the largest and easiest to see. Three much smaller, and harder to see, middle endites lie between the proximal and distal endites. Note the array of medially directed setae on the endites.
The densely setose proximal endite is often referred to as the gnathobase. Its setae form a setal comb, or filter, of finely spaced setae used to filter food particles from the water. The large distal endite may be homologous to the endopod of the ancestral biramous appendage.
The three large exites on the lateral margin of the protopod are easily seen with the dissecting microscope. The proximal and middle exites do not bear setae. The middle exite is theepipod, which was once thought to be a gill although it now appears to be involved in osmoregulation.
The distal exite may be homologous to the exopod of the biramous appendage. It is the only process attached to the protopod by an articulation. It bears long plumose natatory setaeused for swimming. Plumose setae are feather-like to increase their effectiveness as oars. Later you can inspect them with high magnification to see the side branches.
Figure 4. The sixth left phyllopod of Artemia viewed from its anterior surface. Anostraca4La.gif
Feeding
Most anostracans are suspension feeders although a few are carnivores that consume other species of anostracans. A longitudinal, midventral food groove lies between the gnathobases of the phyllopods (Fig 3, 19-12)). The mouth faces the anterior end of the groove. Swimming movements of the phyllopods draw a water current into the groove. The water is then forced laterally through the setal comb on the gnathobases and food particles are prevented from leaving the groove. The food is moved anteriorly in the groove by the setae of the gnathobases. At the anterior end it is entangled in mucus from the labrum and transferred to the mouth by the setae of the first maxillae.
>1a. Place a living active brine shrimp in an 8-cm culture dish or square watch glass with a few milliliters of brine. Add a drop or two of yeast/Congo red suspension and observe the animal with the dissecting microscope. Red food particles quickly accumulate on the setal comb of the phyllopods and in the food groove. This colors the food groove red making it easy to visualize. In a few minutes the red material appears in the anterior gut, rendering it highly visible as well. Set the dish aside and return to it later in conjunction with your study of the digestive system. <
Genital Region
The two segments posterior to the thorax are the genital segments (Fig 1) and bear the external genitalia. Females have a conical pouch called the brood sac (= ovisac) which may contain eggs (Fig 1, 5). Males bear a pair of tubular, retractile penes which can be extended to four times their resting length. The retracted penes are visible posterior to the last pair of phyllopods in male specimens.
Abdomen
The abdomen consists of the six segments posterior to the genital region and is nearly cylindrical (Fig 1). The posterior end of the body is the telson. There is a caudal furca with two short rami on the end of the telson. None of the abdominal segments bears appendages. The anus is located on the telson at the base of the caudal furca (Fig 5).
Internal Anatomy
Living specimens should be used for the study of internal anatomy if possible. Many internal features are not visible in preserved material due to its opacity. Living specimens should be studied with the dissecting microscope in small dishes of chloroformed saltwater (for Artemia) or pondwater (fairy shrimp). This should be supplemented by examination of wholemounts of small specimens with the compound microscope. Instructions for preparing the wholemount are provided in the next section.
Digestive System
The gut is a simple tube extending the length of the animal (Fig 1, 19-11A). Most of it is easy to see in living specimens, especially if the animal has fed recently on a yeast/Congo red suspension. The mouth is located on the ventral midline of the head between the opposing surfaces of the mandibles (Fig 3). The short vertical esophagus extends dorsally from the mouth to open into the stomach above the mouth (Fig 3). The mouth and esophagus are easiest to see in wholemounts of living specimens viewed from the side with the compound microscope. The mouth and esophagus make up the foregut and arise during ontogeny from the stomodeum, an invagination of surface ectoderm.
Figure 5. Left side of the posterior thorax and the abdomen of an immature female Artemia. Anostraca5La.gif
The stomach is an expanded region of the gut in the middle of the head (Figs 3, 6). Two spherical digestive ceca bulge from the anterolateral walls of the stomach (Fig 6).
The intestine is a long tube extending posteriorly from the stomach through the thorax and most of the abdomen (Fig 1, 5). The stomach, ceca, and intestine make up the midgut and are endodermal derivatives. The midgut is the site of enzyme secretion, digestion (hydrolysis), and absorption. It is surrounded by the hemocoel and bathed in blood so that uptake of materials occurs across its thin walls.
The intestine joins the short rectum, or hindgut, in segment 4 of the abdomen (Fig 1, 6). The hindgut, like the foregut, is ectodermal and is lined by a chitinous exoskeleton. It is responsible for formation of fecal pellets and opens to the exterior via the anus between the caudal furcae. The anus is equipped with a sphincter.
The rectum develops from an ectodermal invagination, the proctodeum. Early in development the rectum is not yet continuous with the midgut being separated from it by a partition. Focus with high power on the junction of the mid- and hindguts and see if there seems to be a flow of particles from one to the other.
Figure 6. Dorsal, slightly oblique view of the head of an Artemia protozoea larva. Anostraca6L.gif
>1b. Return to the animal left in the yeast/Congo red suspension earlier (or make such a preparation now). Use the dissecting microscope to observe the shrimp as it swims in its dish and look at the gut. By now the entire length of the digestive tract should be filled with red particles, making it easy to see. It takes only about 15 minutes for yeast to move the length of the gut. Remove the shrimp from the dish and make a wetmount with it for examination with the compound microscope. The regions of the gut should be clearly visible with the compound microscope. Save this slide and refer to it as you study the remaining internal organ systems. Add tapwater to the slide as necessary so that it does not dry out. <
Hemal System
The anostracan hemal system is a good example of the primitive arthropod condition and it is easy to see how it could develop from the dorsal blood vessel of an ancestor.
The heart is a long, median, dorsal tube extending the length of the trunk (Fig 1). It may be visible in living, and sometimes preserved, material with the dissecting microscope but it is best studied with the compound microscope using wholemounts of small living shrimp.
>1c. Prepare a wetmount of a small (about 5 mm), living, unanesthetized brine shrimp. Arrange the shrimp on a slide so you will have a side view of the abdomen and then place a coverslip over the animal. The coverslip will immobilize the specimen but will not immediately stop the heart. Study the animal with the compound microscope and find a place where you can see the abdomen or thorax in side view.
Find the large, tubular intestine, which will be opaque if the animal has been feeding. The transparent, also tubular, heart lies dorsal to the intestine and is easily seen in good preparations (Fig 1). It is surrounded by the pericardial sinus, which is a region of the hemocoel and is not a coelomic space. Anteriorly the heart becomes the short aorta which empties into the anterior hemocoel.
Look for the paired openings, the ostia, in the lateral walls of the heart. Most trunk segments have a pair. The easiest one to see, however, is the large, unpaired terminal ostium at the posterior end of the heart (Fig 1). Ostia are valved pores in the wall of the heart that admit blood from the pericardial sinus into the heart lumen.
Note the beating of the heart. There is no obvious peristalsis and the entire heart appears to contract simultaneously. Anteriorly it opens into the hemocoel, whose spaces extend throughout the tissues of the animal. Elevated pressure in the heart lumen closes the ostia so that blood must exit through the aorta at the anterior end.
Look for small spherical, or ovoid corpuscles and use them as markers to visualize the flow of the blood. The corpuscles tend to move posteriorly in the pericardial sinus outside the heart. Watch closely and you may see some of them pass through ostia in the walls of the heart and then reverse direction and move anteriorly once inside the lumen of the heart. They move in surges associated with contractions of the heart. Entry of corpuscles into the heart is easiest to see in the terminal ostium in the sixth abdominal segment. Sometimes the blood contains hemoglobin in solution in the plasma. Hemoglobin is most likely to be present in animals from water with low dissolved oxygen concentrations. There may be sufficient pigment to impart a pinkish color. <
The heart is surrounded by a special compartment of the hemocoel, the pericardial sinus (Fig 16-7). In living and preserved specimens this area is relatively free of solid tissues and is transparent. It is separated from the rest of the hemocoel, which lies ventral to it, by a perforated, horizontal septum through which blood flows on its way back to the heart. Contractions of the heart force blood out its open anterior end of the aorta into the body hemocoel. Blood flows through the hemocoel and over the tissues while making its way posteriorly. It is aided in its flow by movements of the appendages and their muscles. Blood flows from the body hemocoel into the pericardial sinus through the perforations in the horizontal partition and then passes posteriorly in the sinus and enters the ostia of the heart.
Respiratory and Excretory / Osmoregulatory Systems
Most gas exchange is accomplished across the permeable surfaces of the phyllopods.
The two maxillary glands in the segment of the second maxilla (Figs 1, 3) are usually referred to as excretory organs but, in fact, their role is largely osmoregulatory and they have little to do with the excretion of metabolic wastes. Nitrogen is lost as ammonia across the phyllopod surfaces.
Each maxillary gland consists of an enclosed end sac, derived from a coelomic space, from which a long excretory duct leads to the nephridiopore located on the tiny second maxilla. The duct wraps around the end sac and its coils can be seen through the integument on the side of the head (Fig 1). The gland is surrounded by hemocoel and bathed with blood. The epithelium of the end sac is equipped with podocytes and forms an ultrafiltrate of the blood into the lumen of the end sac. The ultrafiltrate is modified as it passes down the duct to the exterior. Artemia is an efficient osmoregulator and is strongly euryhaline, being tolerant of an impressively wide range of salinities.
Artemia can keep its blood hyposmotic to environments more saline than about 10 parts per thousand. This is something most marine invertebrates cannot do, at least not to the same extent. Artemia drinks brine and actively secretes salts from the maxillary glands, epipods, and gut. The maxillary glands can produce urine four times as salty as the blood. Maintenance of a hyposmotic blood is facilitated by the impermeability of most of the integument. The exoskeleton, with the exception of the epipods, is impermeable to salts. The epipods are major sites of active salt secretion. Artemia belongs to a predominantly freshwater taxon and presumably evolved from freshwater, not marine, ancestors.
>1d. The permeability of the epipods in comparison with the impermeability of the rest of the body surface can be demonstrated with silver nitrate. Remove some small brine shrimp (about 5 mm is a convenient size) from their dish of saltwater. Wash the salt from the outside of the body by placing them in a dish of distilled water for a minute or two. With a pipet, transfer one shrimp to a square watch glass of 0.002M silver nitrate. When the shrimp stops swimming in a few minutes remove it to a microscope slide. Place it ventral side down on the slide so the phyllopods are splayed to the sides and add a coverslip. Examine the specimen with the compound microscope. Silver nitrate reacts with chloride ions to form an insoluble, opaque silver chloride precipitate that turns brown in light. Inspect the shrimp for large dark opaque areas of concentrated silver chloride. Such areas can form only where a permeable integument allows chlorides from the blood to come in contact with silver nitrate. Does the silver chloride seem to be localized or widespread? Pay particular attention to the phyllopods. Does any particular area of the phyllopod appear to be especially permeable? If so, which? Is this observation consistent with what you already know about the functions of the parts of the phyllopod? <
Nervous System
The nervous system is difficult to study in whole specimens, either living or preserved. It consists of a dorsal brain, paired circumenteric connectives, and double, ventral nerve cord with segmental ganglia. The brain is a mass of translucent tissue surrounding the naupliar eye in the dorsal, anterior part of the head. You may be able to see it in living specimens.
The sensory system includes the median naupliar eye which appears in the earliest larval instar and persists throughout life. It consists of three black pigment cups. Two cups face laterally and one points ventrally. Two stalked, lateral compound eyes, each composed of numerous black ommatidia are also present.
Reproductive System
The gonads of both sexes are paired tubes located dorsolaterally in the posterior thorax and anterior abdomen (Fig 1). They may be faintly visible as transparent, elongate sacs beside the intestine. They are easiest to see in wetmounts of small, living specimens.
Reproduction
During mating the male approaches the dorsal side of the female and holds her with his enlarged second antennae. The male twists his body around the female, inserts the penes into the brood pouch, and deposits sperm. The partners remain coupled for several hours during which copulation may occur every few minutes. Eggs are released unto the brood pouch where they are fertilized and covered with a shell.
Two kinds of eggs are produced. One has a thin shell and hatches in the brood pouch. The other, known as the resting egg, has a heavy shell and can remain viable for several years out of water and then hatch when immersed in saltwater. This second type of egg is collected and sold by supply houses and aquarium shops. Both egg types hatch into nauplius larvae.
Appendages
" Return now to the postponed study of the thoracic appendages. Use your nadeln and fine forceps to remove a phyllopod from your anesthetized specimen and make a wholemount with it. Select an appendage from near the middle of the thorax for this purpose. Examine the slide with the compound microscope. Return to the description of thoracopods which appeared earlier in this exercise and find the structures you were unable to see with the dissecting microscope. Be sure to examine the plumose swimming setae of the endopod and consider how their structure is adapted for swimming.
>1e. Make a wholemount of a phyllopod if you have not already done so. Use 100 and 400X of the compound microscope to compare the many types of setae characteristic of the different areas of the phyllopod.
The setae of the exopod (distal exite) are large, strong and plumose, or pinnately branched. Plumose setae have lateral branches from the central shaft and resemble a feather. These are swimming setae, or natatory setae, that function as paddles to increase the surface area of the appendage and enhance its effectiveness in swimming. The lateral branches of the seta increase the effective surface area of the seta and make a little oar of it.
The setae of the endopod (distal endite) are short, fairly stout, and finely serrate. These are scraping setae used to scrape algae from hard substrata.
Setae on the three middle endites are similar but are heavier, generally longer and more coarsely serrate. They are also scraping setae and these three endites are sometimes called "claws".
The setae on the proximal endite are fine and delicate. They are very finely plumose although that is not apparent unless the light is perfectly adjusted. These are the filter setae of thesetal comb used to remove food from the water as it exits the sides of the food groove. Does the structure of these different kinds of setae seem to be adapted to their functions? <
Behavior
Adult anostracans swim constantly using the phyllopodous thoracic appendages. Waves of motion pass along the series of phyllopods to draw water and food particles into the food groove and to create an effective stroke with the swimming setae so that locomotion and feeding are accomplished simultaneously. Artemia also feeds by scraping algae from hard surfaces. Anostracans can make sudden quick moves by flexing the abdomen. The caudal furca is also equipped with plumose swimming setae.
>1f. With a dissecting microscope and incident illumination observe a living, active adult brine shrimp swimming in a dish of water. Note the orientation of the animal in the water. The preferred orientation is with the dorsum down butArtemia can swim right side up also. The filter-feeding mechanism works best when upside down. Which appendages are used for locomotion? Do the antennae seem to be involved in swimming? Does the animal ever stop swimming? Do you see any evidence of feeding by scraping the bottom? Watch for movement resulting from flicking the abdomen. How does it differ from motion produced by phyllopods? <
Artemia, with its three eyes, is sensitive to light intensity and exhibits highly variable responses to light. In general, Artemia is positively phototactic at low light intensities and photonegative at medium and high intensities. The response varies, however, depending on the physiological condition of the animal, wavelength, age, salinity, pH, and metabolic condition.
>1g. Place a dish containing larvae in a part of the room where it will receive uneven illumination. Leave the dish for 15 minutes or so and then observe the distribution of shrimp. Do they seem to be clustered in any particular region of the dish? How does this relate to the light source? You may want to design a series of more carefully controlled experiments to determine the effect of light intensity, life history stage, salinity, wavelength, or temperature. <
Most animals exhibit what is called the "dorsal light reaction" in response to the sun's rays by maintaining their dorsal surface up. A few animals, such as Artemia, backswimmers (hemipterous insects), and the fish louse, Argulus (a crustacean), reverse this response and exhibit a "ventral light reaction" and keep the ventral surface pointed toward light. Consequently, brine shrimp (and fairy shrimp) normally swim upside down (ventral side up), because in nature the light is overhead. A brine shrimp in a dish on the stage of a dissecting microscope withsubstage illumination, however, may reverse its orientation and swims with the ventral side down.
>1h. Place a brine shrimp or fairy shrimp in a small culture dish and put it on the stage of the dissecting microscope with the incident lamp on and the substage lamp off. Observe the swimming orientation of the animal. Turn the incident lamp off and the substage (transmitted) lamp on. Do this several times noting the response of the animal. <
Larvae
A laboratory culture of Artemia will provide representatives of the major larval stages of a typical crustacean life cycle. Artemia requires about 14 molts to reach its terminal size and achieves sexual maturity in about 12 molts. The stages between successive molts are instars. Artemia larval stages are the nauplius, which includes a metanauplius, and the zoea. No megalops is present. A nauplius is a crustacean larva that swims with head appendages, whereas a zoea is an older larva that swims with thoracic appendages. The megalops, which is older still, swims with abdominal appendages.
In Artemia the larvae hatch with few segments and gradually increase the number to 19 with successive molts. The mitotically active teloblast areas in the telson add new segment buds with each molt until the characteristic number for the species is achieved. New limb buds appear on existing segments with each molt. The 19 trunk segments of Artemia are added in groups, rather than one with each molt.
Nauplius
Use a fine pipet to select several larvae of each of as many different sizes as possible from the laboratory culture. Transfer them to a small (6-cm) culture dish of chloroform-saturated saltwater or add a few drops of chloroform to the culture dish and wait for the larvae to become anesthetized. When the larvae stop moving, transfer some of the smallest to a glass slide and make a wetmount with them. Try to arrange the larvae so some have the venter facing up and others the dorsum. Support the coverslip with wax feet and press it gently against the larvae but do not crush or distort them. Refer to the Techniques chapter for instructions on making wax feet.
With the compound microscope find one of the smallest larvae, which should be a nauplius (Fig 7, 19-8). Artemia hatches as a nauplius with a short body consisting of the first three head segments and a short trunk but with no external segmentation.
Figure 7. An Artemia nauplius larva. A. Dorsal, B. Ventral. Anostraca7La.gif
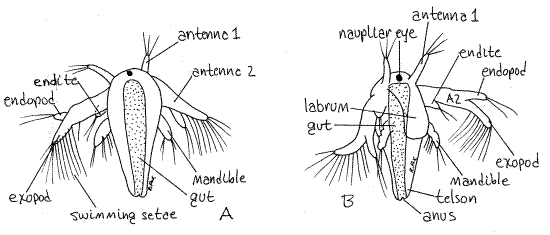
Stored yolk gives the body an orangish color and renders it nearly opaque. Due to this opacity it is difficult to discern internal structures. (The interior is much easier to see in an older metanauplius in which the yolk reserves have been exhausted and the tissues are transparent.) The nauplius is lecithotrophic, living off its yolk reserves without feeding.
Find the dark, red or black, median naupliar eye at the anterior end of the head. It consists of three pigment cup ocelli. The brain may be visible surrounding the eye in older, more transparent embryos. No carapace is present in adult or larval anostracans.
The anteriormost appendages are the small uniramous first antennae. The second antennae are the largest appendages of the nauplius and are the principal swimming organs (Fig 7). They are equipped with long natatory swimming setae and are biramous. The chief swimming setae are on the exopod. The endopod is smaller and lacks the row of evenly spaced swimming setae.
The third pair of appendages is the uniramous mandibles. The labrum is a large, thin, ventral fold of body wall arising between the second antennae and the mandibles, immediately anterior to the mouth (Fig 7B). It is not a segmental appendage.
The posterior end of the body is the telson (Fig 7). The telson contains the teloblast areas that produce the mesoderm from which new segments are fashioned. Buds produced at the anterior edge of the telson differentiate into new segments. The youngest segments are closest to the telson and the oldest are anterior.
>1i. With the dissecting microscope observe a few active Artemia larvae in a small culture dish of salt solution or seawater. Watch one swim and try to see which appendages it uses. Swimming with a single pair of appendages is erratic and jerky. Compare this motion with the smooth swimming of adults. Adult swimming utilizes 11 pairs of appendages and is much smoother. <
Metanauplius
Try to find a small transparent larva about the size of the opaque, orange nauplii but with a longer trunk. Such a larva will be a metanauplius. The metanauplius is a slightly older larva but, since it swims with its head appendages, is still considered to be a nauplius.
The metanauplius stage includes several molts and instars and lasts for several days. It ends when the anterior thoracic limbs become functional in the tenth instar. The metanauplius begins feeding when its yolk reserves are exhausted.
The metanauplius has an unsegmented head to which is attached an elongate thorax. The anterior end of the thorax is visibly segmented. The internal organs of these slightly older larvae are easier to see than are those of the nauplius.
The gut extends from the head posteriorly to the telson. The mouth is on the ventral surface of the head, between the bases of the mandibles, under the labrum but you probably cannot see it. The stomach is wider than the rest of the gut and is situated dorsal to the mouth to which it is connected by a vertical esophagus. Two diverticula, the digestive ceca, extend anterolaterally from the stomach.
The intestine extends posteriorly from the stomach. In the nauplius it is relatively short but it is long, narrow, and getting longer in the metanauplius. The posterior end of the gut is hindgut, or rectum, and it differs from the midgut in appearance. Its walls are nearly colorless whereas those of the midgut are reddish brown with the remaining yolk. The rectum opens to the exterior via the anus at the end of the telson.
Sessile lateral eyes appear in the metanauplius or zoea. They require several molts to develop their stalks.
Zoea
The zoea stage, consisting of many instars, follows the metanauplius. The zoeal sequence begins with a long transition period (known as the protozoea) in which both the head and thoracic appendages are involved in swimming.
Loss of its swimming setae and cessation of swimming movements by the second antennae signals the beginning of the true zoeal stage. (In Artemia this period is sometimes referred to as the postlarva. The larva resembles the adult but is smaller.) During this period the limb primordia continue developing into functional limbs and the animal grows to its adult size. The animal becomes an adult when all appendages are present, complete, and functional but molting continues every few days through an adult life of several months.
>1j. If protozoea or zoea are available, place a dish with a few larvae on the stage of a dissecting microscope and watch them swim. Compare the swimming with that of the nauplius. The swimming motion becomes progressively smoother as more and more pairs of phyllopods replace the single pair of antennae. <
References
Abbott DP . 1987. Observing Marine Invertebrates. Stanford Univ. Press, Stanford. 380p.
Anderson DT. 1967. Larval development and segment formation in the branchiopod crustaceans Limnadia stanleyana King (Conchostraca) and Artemia salina (L.) (Anostraca). Aust. J. Zool. 15:47-91.
Bond RM . 1937. A method for rearing Artemia salina, in Needham, J. G. et al., Culture Methods for Invertebrate Animals. Comstock, Ithaca. 205-206.
Brown FA . (ed) 1950. Selected Invertebrate Types. Wiley, New York. 597p.
Browne RA. 1993. Sex and the single brine shrimp. Natural History, 102(5):34-39.
Bullough WS . 1958. Practical Invertebrate Anatomy (2nd ed). MacMillan, London. 483p.
Green J. 1981. Crustaceans, in Dales, R. P. (ed) Practical Invertebrate Zoology. MacMillan, London. pp. 261-302.
Heath H. 1924. The external development of certain phyllopods. Jour. Morphol. 38:453-483.
Linder F. 1941 (1946?). Contributions to the morphology and the taxonomy of the Branchiopoda Anostraca. Zool. Bidrag. Uppsala 20:101-302.
Martin JW. 1992. Branchiopoda, pp 25-224 in Harrison, F. W. & A. G. Humes (eds.). 1992. Microscopic Anatomy of Invertebrates vol. 9 Crustacea . Wiley-Liss, New York.
Moore WG. 1957. Studies on the laboratory culture of Anostraca. Trans. Am. Micros. Soc. 76:159-173.
Pardi L, Papi F. 1961. Kinetic and tactic responses, in T. H. Waterman, The Physiology of Crustacea II. Academic Press, New York. p 365-399.
Pennak RW . 1989. Fresh-water Invertebrates of the United States, 3 ed. Wiley, New York. 628p.
Personne G. et al. (eds.). 1980. The Brine Shrimp , Artemia, vols 1-3. Universa Press, Wetteren, Belgium.
Ruppert EE, Fox RS, Barnes RB. 2004. Invertebrate Zoology, A functional evolutionary approach, 7 th ed. Brooks Cole Thomson, Belmont CA. 963 pp.
Walley LJ. 1969. Studies on the larval structure and metamorphosis of Balanus balanoides (L.). Philos. Trans. Roy. Soc. London, Ser B 256:237-280.
Williamson, D. I. 1982. Larval morphology and diversity, pp. 43-110 in D. E. Bliss (ed), The Biology of Crustacea vol 2. Academic Press, New York.
Supplies
Dissecting microscope
Compound microscope
8-cm culture dish or square watch glass
Yeast / Congo red suspension (See Supplies and Recipes chapter)
Slides and coverslips
Beeswax
plastic Pasteur pipets
chloroform-saturated seawater
iodine-free salt (e.g. ice cream salt)
distilled water
5-10 ml 0.002M silver nitrate
Brine shrimp (Artemia) eggs or colony
It is convenient to have a self-perpetuating colony in which nauplii, zoea, postlarvae, and adults are always available. Alternatively, the necessary life history stages can be produced by adding eggs to saltwater 48 hours, 96 hours, 1 week and three weeks prior to the day of the laboratory in which they are needed.
Brine Shrimp Cultures
Brine shrimp are easily reared in a salt solution (brine) of almost any salinity. A suitable concentration can be achieved using 40 g ofnon-iodized salt (e.g. ice cream salt or non-iodized table salt) per liter of chlorine-free fresh water. Use pond, spring, distilled, or aged tapwater. The brine should be in a shallow, non-metallic (i.e. glass) pan or dish with a large exposed surface area and shallow depth (about 2-3 cm). Do not aerate.
With a lab marker, mark the original level of the brine on the side of the container and add distilled water as needed replace evaporative losses and maintain this level. Do not replace evaporative losses with more brine. Do not cover the container as this restricts the oxygen supply.
To have adults and all larvae stages on the day of the laboratory exercise, begin about 3-4 weeks prior to the day for which the animals are needed. Add a tiny pinch of Artemia eggs to the brine in the shallow dish. Add an additional tiny pinch of eggs every 4 days until about a week before the animals are needed and then add a tiny pinch of eggs daily. Eggs will begin hatching about 24-48 hours after being placed in the brine.
The second day after the first eggs hatch, a feeding regimen should be instituted and maintained thereafter for the life of the culture. Prepare a suspension of one package baker’s yeast in 100 ml of fresh water and store it covered, in a refrigerator (See Supplies and Recipes chapter). Each day, use a pipet to add enough of the suspension to render the water in the cultureslightly cloudy. Do not overfeed, do not add too many eggs, and do not aerate. Keep the yeast suspension refrigerated. Do not add more yeast than the shrimp can remove before the next feeding. The water should never become opaque rather should be transparent or nearly so.
The population density must be low enough that sufficient oxygen can enter the water by diffusion from the surface. Artemia larvae are sensitive to increased carbon dioxide concentrations but under the conditions described can be reared without artificial aeration. Aeration damages the older, more fragile larvae and adults but does not harm nauplii and metanauplii.
Larvae can be reared to maturity by this method and, in fact, continuous cultures can be maintained with the adults of the first generation producing eggs for subsequent generations. Nauplii will be available 24-48 hours after the eggs are added to the water. Other larval stages will follow in turn. Adults will appear in about three weeks and will be seen mating (swimming in tandem) shortly after. Once adults appear, it is no longer necessary to add eggs. Mature females produce thin-walled eggs which hatch in the brood chamber and are released as larvae to perpetuate the colony.
If only nauplii or metanauplii are needed, feeding is not necessary and the culture can be in a deeper container, such as a gallon jar, and aerated with an airstone. Under these conditions the population density can be vastly increased. Start such a high density culture (aerated) with 5 ml dry eggs per 4 liters of saltwater 36-48 hours prior to the time the larvae are needed. A culture with this density must be aerated vigorously. Aeration does not damage the nauplii but will kill the more delicate older instars and adults.
Sources
Artemia resting eggs are available from biological supply houses, pet shops, and aquarium stores.
Living Eubranchipus are available Apr 1 to May 15 from Nebraska Scientific, 3832 Leavenworth St., Omaha, Nebraska 68105.
Preserved Eubranchipus are available from Carolina Biological and Ward's Natural Science.
Prepared wholemount slides of small fairy shrimps are available from Carolina Biological and Triarch.
Prepared slides of Artemia nauplii are available from Triarch.
Ward's markets, under the name "Living Fossil" culture, vials of soil from the bottom of temporary ponds that contain eggs of fairy shrimp, tadpole shrimp, and cladocerans. Adults can be reared from this soil.