Invertebrate Anatomy OnLine
Acheta domestica ©
European House Cricket
29may2007
Copyright 2001 by
Richard Fox
Lander University
Preface
This is one of many exercises available from Invertebrate Anatomy OnLine , an Internet laboratory manual for courses in Invertebrate Zoology. Additional exercises can be accessed by clicking on the links to the left. A glossary and chapters on supplies and laboratory techniques are also available. Terminology and phylogeny used in these exercises correspond to usage in the Invertebrate Zoology textbook by Ruppert, Fox, and Barnes (2004). Hyphenated figure callouts refer to figures in the textbook. Callouts that are not hyphenated refer to figures embedded in the exercise. The glossary includes terms from this textbook as well as the laboratory exercises.
Systematics
Arthropoda P, Mandibulata sP, Tracheata, Hexapoda SC, Insecta C, Dicondylia, Pterygota, Metapterygota, Neoptera, Orthopterodea SO, Orthoptera O, Ensifera sO, Grillidea iO, Grylliodea SF, Gryllidae F, (Fig 16-15, 20-14, 20-15, 21-23)
Arthropoda
Arthropoda, by far the largest and most diverse animal taxon, includes chelicerates, insects, myriapods, and crustaceans as well as many extinct taxa. The body is segmented and primitively bears a pair of jointed appendages on each segment. The epidermis secretes a complex cuticular exoskeleton which must be molted to permit increase in size. Extant arthropods exhibit regional specialization in the structure and function of segments and appendages. The body is typically divided into a head and trunk, of which the trunk is often itself divided into thorax and abdomen.
The gut consists of foregut, midgut, and hindgut and extends the length of the body from anterior mouth to posterior anus. Foregut and hindgut are epidermal invaginations, being derived from the embryonic stomodeum and proctodeum respectively, and are lined by cuticle, as are all epidermal surfaces. The midgut is endodermal and is responsible for most enzyme secretion, hydrolysis, and absorption.
The coelom is reduced to small spaces associated with the gonads and kidney. The functional body cavity is a spacious hemocoel divided by a horizontal diaphragm into a dorsal pericardial sinus and a much larger perivisceral sinus. Sometimes there is a small ventral perineural sinus surrounding the ventral nerve cord.
The hemal system includes a dorsal, contractile, tubular, ostiate heart that pumps blood to and from the hemocoel. Excretory organs vary with taxon and include Malpighian tubules, saccate nephridia, and nephrocytes. Respiratory organs also vary with taxon and include many types of gills, book lungs, and tracheae.
The nervous system consists of a dorsal, anterior brain of two or three pairs of ganglia, circumenteric connectives, and a paired ventral nerve cord with segmental ganglia and segmental peripheral nerves. Various degrees of condensation and cephalization are found in different taxa.
Development is derived with centrolecithal eggs and superficial cleavage. There is frequently a larva although development is direct in many. Juveniles pass through a series of instars separated by molts until reaching the adult size and reproductive condition. At this time molting and growth may cease or continue, depending on taxon.
Mandibulata
Mandibulata includes arthropods in which the third head segment bears a pair of mandibles. As currently conceived this taxon includes myriapods, hexapods, and crustaceans. Appendages may be uni- or biramous and habitats include marine, freshwater, terrestrial, and aerial.
Tracheata
Myriapods and hexapods share tracheae and a single pair of antennae and are sister taxa in Tracheata. Crustaceans, which have gills and lack tracheae, are excluded and form the sister group.
Hexapoda
The body is divided into three tagmata; head, thorax, and abdomen. Appendages are uniramous and a single pair of antennae is present. Three pairs of legs and two pairs of wings are found on the thorax of most adults. Hexapod legs are uniramous although there is increasing evidence that they evolved from multiramous appendages of their ancestors. Gas exchange is accomplished by trachea. Excretory organs are Malpighian tubules and the end product of nitrogen metabolism is uric acid. There is relatively little cephalization of the nervous system. Insects are gonochoric with copulation and internal fertilization.
Insecta
Most hexapods are insects. A few hexapod taxa (orders) lack wings and have primitive mouthparts recessed into the head and belong to Entognatha, the sister taxon of Insecta. Insects have ectognath mouthparts and the adults (imagoes) of most taxa have wings.
Pterygota
Pterygotes are the winged insects. These insects are derived from a winged common ancestor. Adults of most taxa have wings although they have been lost in some.
Orthoptera
Orthoptera includes some 20,000 species of grasshoppers, crickets, locusts, and katydids. These are relatively large insects with an enlarged pronotum. The hind femora are large and adapted for jumping. Females have a large ovipositor but male genitalia are not visible externally. Most are herbivores.
Laboratory Specimens
This exercise is based on crickets in the similar genera Acheta and Gryllus and either can be used. Gryllus pennsylvanicus, the (North American native) northern field cricket, andAcheta domestica, the (introduced) European house cricket, are the most abundant crickets in the eastern United States and are especially common around and often inside houses. The two species are similar and belong to the same subfamily (Gryllinae), which comprises the house and field crickets. Acheta domesticus is pale yellowish-brown and has conspicuous, dark, transverse bands across the head. Acheta is reared commercially for fishing bait and living specimens are readily available at negligible cost in bait shops.
Each student should study adult male and female crickets. Females can be recognized by the long, needlelike ovipositor extending posteriorly from the abdomen whereas males lack this organ. Both sexes have two long posterior cerci which should not be confused with the ovipositor. Females have three long posterior processes whereas males have only two. Crickets are paurometabolous insects in which juveniles are miniature replicas of adults. Imagoes, as adult insects are known, are recognizable by their fully developed wings. Juveniles, known asnymphs, develop short, thick, fleshy wing pads in older instars but do not have functional wings.
Anesthetize the two crickets in a jar containing a ball of cotton slightly damp with chloroform or ether. If desired the insects can be killed by using ethyl acetate instead of anesthetic. Remove the insects as soon as they become inactive, returning them to the jar as necessary to reanesthetize them during the study. Five to ten seconds exposure to carbon dioxide (from dry ice or a cylinder) is also an effective anesthetic and, in fact, is preferable if physiological work is anticipated. Alternatively, the insects can be sacrificed with fumes from a cotton ball dampened with a little ethyl acetate. Preserved specimens can also be used but there is usually no reason to do so and anesthetized living, or freshly sacrificed, specimens are far superior.
Cricket anatomy is very much like that of grasshoppers and locusts, all of which belong to the same order. If crickets are not available, or a larger animal is desired, any of these could be used for this exercise if one allows for some variation from the cricket plan.
External Anatomy
Place the crickets in a small, dry dissecting pan or culture dish. (An anchovy fillet can with a wax bottom makes a good dissecting pan for crickets.) Study the external anatomy using the low power of the dissecting microscope. Begin with either sex and switch from one to the other as appropriate.
Tagmata
The arthropod body is composed of a linear series of segments, some or all of which bear a pair of jointed segmental appendages. Most arthropods, including insects, areheteronomous , with segments and appendages variously modified and grouped in regions, or tagmata, specialized for different functions. The homonomous condition, with all segments and appendages alike over the length of the body, is primitive but, among Recent taxa, is approached only by a few crustaceans.
The three insect tagmata are the anterior head, middle thorax, and posterior abdomen (Fig 1, 21-1C). The head, which shows no external signs of segmentation, bears the eyes, antennae, mouth, and mouthparts. Its chief functions are sensory reception and feeding. The thorax, whose principal function is locomotion, is larger and bears three pairs of legs and two pairs of wings (wings are present only in adults). The abdomen is the largest tagma and is conspicuously segmented. It houses most of the digestive, excretory, and reproductive viscera and its appendages, when present, are specialized for copulation or oviposition. Most abdominal segments lack appendages and those present are highly modified.
Integument
The arthropod body is covered by an elaborate, well-developed integument consisting of a monolayered epidermis and a complex outer exoskeleton, or cuticle. The epidermis lies under the cuticle, which it secretes. Some regions of the cuticle are hardened, or sclerotized, by tanning to form stiff plates known as sclerites.
Figure 1. View of the left side of a female Acheta. Abdominal segments and tarsomeres are numbered. Orthop8La.gif
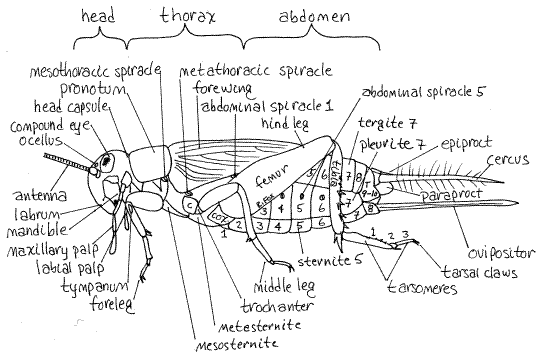
Adjacent sclerites may be connected by areas of soft, unsclerotized cuticle known as articular membranes or they may be rigidly fused together. A typical arthropod segment is surrounded by four sclerites (Fig 16-1B). These are the dorsal tergite, ventral sternite, and a pleurite on each side but there are many variations on this plan.
Head
Head Capsule
The head is enclosed in a heavily sclerotized, rigid, exoskeletal head capsule, or cranium, composed of the sclerites of the segments that make up the head (Figs 1, 2, 21-1A,B). The sclerites are fused firmly together and are not recognizable as separate entities. The four pairs of head appendages articulate with the capsule.
Eyes
Two lateral compound eyes are located dorsolaterally on the head. Examine one with the highest power of the dissecting microscope to see that it is composed of abundant small photoreceptive units, or ommatidia. The surface of the eye is a specialized, transparent part of the exoskeleton divided into tiny hexagonal corneas, one for each ommatidium. The cornea is the only part of the ommatidium visible from the outside surface. The compound eyes are innervated by the protocerebrum as they are in other arthropods.
Anteriorly on the head capsule are three small, white simple eyes, or ocelli, each of which is a single photoreceptor that functions in evaluation of light intensity (Fig 2, 21-1A).
Figure 2. En face view of the head of the cricket, Acheta. Orthop9L.gif
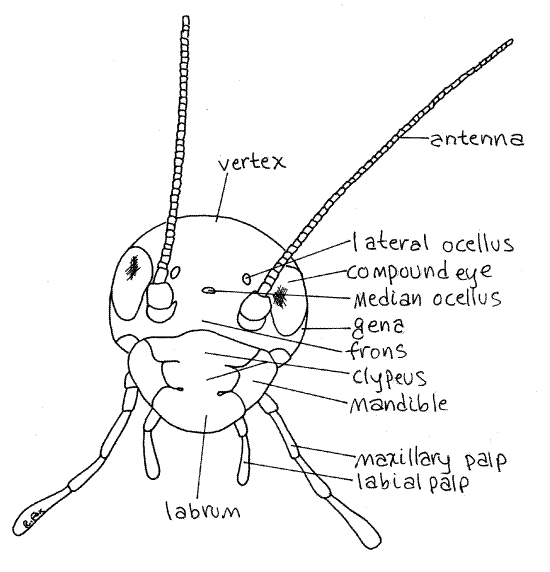
Head Regions
The head capsule is divided into regions, each representing a sclerite, and each with a name. The front of the head, or the region anterior to the compound eyes, is the frons (Fig 2). The clypeus (CLIP ee us) is a platelike sclerite ventral to the anterior edge of the head capsule (Fig 2, 21-1A). It appears to be a ventral extension of the frons but is separated from it by a transverse groove, the sulcus. Each lateral edge of the clypeus bears a deep notch. Ventral to and posterior to the compound eye on each side of the head is the cheek, or gena. The top of the head is the vertex. The posterior wall of the head capsule is the occiput. The occipital foramen (= foramen magnum) is a large hole in the center of the occiput. Through it pass the organ systems, such as nerve cord, gut, and musculature, connecting the head with the body. The foramen is filled with soft tissue and is not visible externally although you can see its circular outline.
Antennae
Insects have four pairs of head appendages. Two long, many-jointed, sensory, filamentous antennae are attached to shallow sockets in the antero-dorsal corners of the head (Fig 2). The antennae are the anteriormost segmental appendages of the insect head. They are innervated by the deutocerebrum as are the first antennae of crustaceans.
Mouthparts
The mouthparts surround the mouth on the ventral surface of the head. The mandibulate mouthparts of orthopterans are adapted for biting and chewing. Mandibulate mouthparts are the ancestral condition from which the specialized types of other insects were derived. In Orthoptera the mouthparts are below the head and point downward, a condition referred to as hypognathous. The mouthparts surround a chamber, the preoral cavity, that precedes the mouth which opens from it. Beginning anteriorly, find the mouthparts.
To best observe the mouthparts without removing them, place the insect on its back, dorsal side down, in a small dissecting pan. Push a # 1 stainless steel insect pin through the neck, into the occipital foramen, through the vertex, and then into the wax of the pan. This will hold the head stationary while you simultaneously use fine forceps and a microneedle to manipulate the mouthparts.
Labrum
The labrum, or upper lip, is attached to the ventral edge of the head capsule on the anterior face of the head (Figs 3, 1, 2). It is unpaired and is the first of the mouthparts. The labrum covers and protects the more posterior mouthparts and is the anterior border of the preoral cavity.
Figure 3. The labrum of the cricket, Acheta. Orthop10L.gif
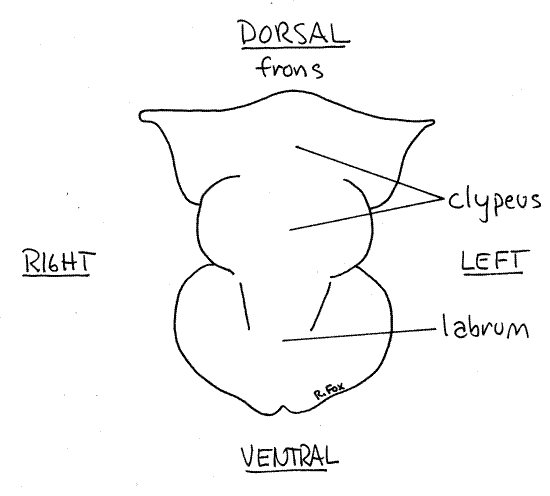
Lift the labrum to demonstrate its mobility and to reveal the preoral cavity and the remaining mouthparts. The labrum is usually considered to be an outgrowth of the head capsule rather than an appendage. It is, however, equipped with muscles, is movable, and is innervated by the tritocerebrum and is thought by some entomologists to be derived from the fused right and left appendages of its segment.
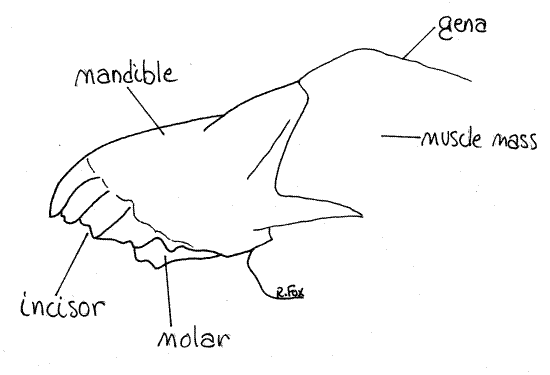
Mandible
Immediately posterior to the labrum is a pair of unjointed mandibles (Figs 4, 1, 2, 21-1A,B). The mandibles of crickets are large, massive, and adapted for biting and chewing. Their dark, heavily sclerotized, and strongly toothed median edges are apparent when the labrum is moved aside. If your specimen is alive, you may get to watch the mandibles move naturally or you can move them yourself, noting that their motion is restricted to the transverse plane. Such joints, having two condyles (points of articulation), limit motion to a single plane (like your knee or elbow). The mandible articulates with the ventral edge of the head capsule by a dicondylic joint. The two mandibles lie on either side of the mouth and, along with the maxillae, form the lateral walls of the preoral cavity from which the mouth opens. The toothed cutting surface of the mandible includes a distal incisor of sharp, shearing teeth and a proximal molar of smaller grinding teeth (Fig 4).
Figure 4. The mandible of the cricket, Acheta. Orthop11L.gif
Maxilla
The maxillae are posterior to the mandibles (Figs 5, 1, 2, 21-1A,B) and articulate with the side of the head capsule. The maxillae are paired and jointed and each bears a large, sensory, multiarticulate maxillary palp.
Each maxilla consists of two basal articles. The proximal article is the cardo, which articulates with ventral edge of the gena (Fig 5). The distal article is the stipes, which articulates with the cardo. From the stipes arise three distal processes. The lateralmost of these is the sensory maxillary palp which extends away from the head and is easy to see. The middle process is the spoon-shaped galea. The median process is the toothed and sclerotized lacinia. The galea and lacinia curve medially, just posterior to the incisor of the mandible.
Labium
The labium, or lower lip, is the posteriormost head appendage and consists of the fused right and left second maxillae of the ancestor (Fig 6, 21-1A,B). Each side of the labium bears a sensory labial palp which resembles and is serially homologous to the maxillary palps. The labium closes the preoral cavity posteriorly.
Figure 5. The maxilla of the cricket, Acheta. Orthop12L.gif
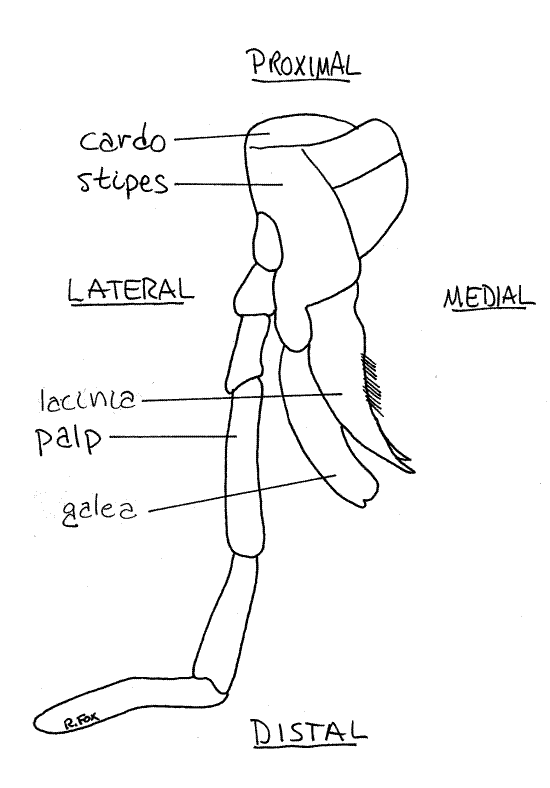
Figure 6. The labium of the cricket, Acheta. Orthop13L.gif
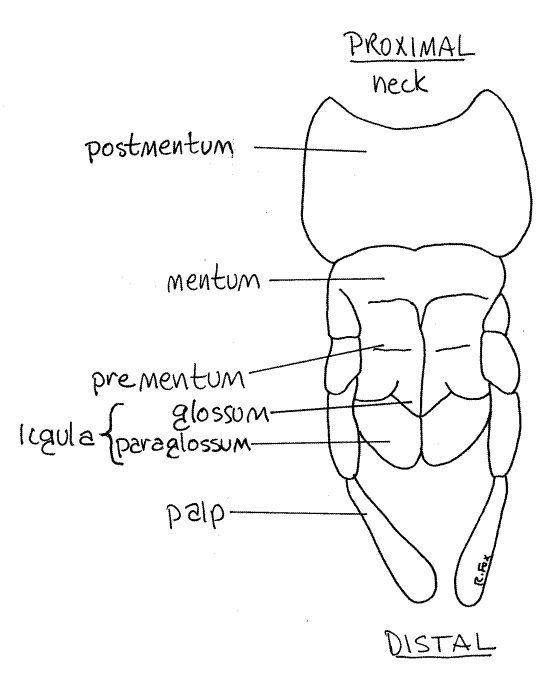
The basal portion of the labium is the postmentum. Attached to the distal edge of the postmentum is the mentum. The central region of the labium is the prementum. It bears the palps and ligulae. The anterior edge of the prementum is deeply cleft medially, reminding us that the labium is formed evolutionarily of a pair of fused appendages. Distally the prementum bears two slightly cupped ligulae side by side on either side of the ventral midline. Each ligula is composed of a medial glossum and a lateral paraglossum.
Hypopharynx
Push the labium posteriorly and look into the preoral cavity. The mandibles and maxillae form the sides of the cavity. Inside the cavity is the large, soft, bulging hypopharynxextending from the ventral wall of the head immediately posterior to the mouth. The hypopharynx is an unpaired fold of the body wall and is not a segmental appendage. It divides the preoral cavity into an anterior cibarium, from which the mouth opens, and a posterior salivarium, into which the duct of the salivary glands opens (Fig 21-7).
The mouth is on the ventral surface of the head between the bases of the hypopharynx and labrum but it is difficult to hold the appendages out of the way so you can get a good look at it. If you pull posteriorly on the labium, the hypopharynx will move posteriorly out of the way. At the same time pull anteriorly on the labrum and the mouth will be revealed in the cibarium between it and the hypopharynx.
Neck
Head and thorax are connected by a short narrow cervix , or neck, whose integument is unsclerotized, soft, and flexible (Fig 1). The cervix is easy to see if you gently push the head aside and look between it and the thorax. A few small, lightly sclerotized plates, the cervical sclerites, are present in its integument.
Thorax
The insect thorax consists of three segments and bears three pairs of legs and, in the adults of most taxa, two pairs of wings. The powerful musculature to operate these ten locomotory organs is housed in the thorax. If you have not already done so, remove the pin holding the insect to the wax.
Each thoracic segment bears a pair of legs, which are segmental, jointed appendages. In most adult insects the posterior two thoracic segments each bear a pair of wings. The wings are very thin folds of the body wall and are not jointed and are not segmental appendages.
The three thoracic segments are, in order from anterior to posterior, the prothorax, mesothorax, and metathorax. Dorsally and laterally the prothorax is covered by a large archlike tergite called the pronotum (Fig 1, 21-1C). The inconspicuous prosternite, on the ventral surface of the prothorax, is much smaller and is not heavily sclerotized or pigmented. The pleurites of the prothorax, known as propleurites, are small and hidden by the overhang of the pronotum. (The tergites of the insect thorax are called nota, rather than tergites. The sternites and pleurites do not have special names.)
The forelegs attach to the prothorax. Note the flexible, unsclerotized cuticle forming an articular membrane around the base of the leg. Rotate the leg around the articulation to demonstrate its mobility.
The mesothorax and metathorax bear the middle and hindlegs respectively (Fig 1) and each also bears a pair of wings. The wings hide the dorsal surface of these segments. Because they bear wings and house the flight muscles these two segments together are known as the pterothorax (pter = wing). The wings of the mesothorax are the forewings, or wing covers, and those of the metathorax are the hindwings. In Acheta the delicate and nonfunctional hindwings are often damaged or missing entirely. Lift the wings so you can see the pterothorax clearly.
Dorsally each of these two segments is covered by its own sclerotized tergite, known respectively as the mesonotum and metanotum. The mesonotum is small and hidden by the posterior edge of the pronotum. The large metanotum is divided into three plates. Ventrally these segments are covered by the mesosternite and metasternite, which are much larger than the prosternite. The pleurites of these two segments bear small plates.
Primitively, each segment bears a pair of lateral openings, the spiracles, for the intake of air into the respiratory system. In modern insects this plan is altered and some segments lack spiracles. For example, the head and posteriormost abdominal segments have no spiracles and there the three thoracic segments share two pairs of spiracles. The prothorax has no spiracles. The mesothoracic spiracle is a large opening between the prothorax and mesothorax on the dorsal end of a swelling just posterior to the base of the foreleg and on the anterior edge of the mesothorax (Fig 1). It is covered by a soft bulging operculum that must be reflected to reveal the spiracle. It may gape open of its own accord. The metathoracic spiracle is a slit in the membrane between the metathorax and mesothorax. Note that theopening is guarded by two movable lips, or valves, whose function is to reduce water loss from the trachea.
Legs
Look at one of the middle (mesothoracic) legs (Fig 1, 21-1E). This appendage, which is typical of insect legs, is composed of a linear series of six articles. Note that while most of its cuticle is rigid and heavily sclerotized, the parts between articles are unsclerotized and flexible to allow movement of the articles. The articles bear cuticular spines and setae of various sizes and designs.
The first (proximal) article is the coxa. It is short and articulates by an articular membrane with the pleurite and sternite of the segment. The short, narrow trochanter is articulated with its distal end. The third article is the long femur. The tibia, which is also long, articulates with the distal end of the femur.
The next article, the tarsus, is composed of three parts, or tarsomeres. The final article of the leg is the pretarsus. It attaches to the distal end of the tarsus and bears a pair of tarsal claws. The claws are conspicuous but the pretarsus itself is small and hard to see.
The articulation between the femur and tibia is a dicondylic hinge joint, similar to the one in your elbow or knee, that restricts motion to a single plane. Move the tibia with respect to the femur and note its limited range of motion. Compare the femur/tibia articulation with that made by the coxa and its socket. The latter allows a great range of motion and is equivalent to a ball and socket joint such as that found in your shoulder or hip.
The forelegs are nearly identical to the middle legs except that the proximal end of the tibia of the foreleg (of adults) bears an oval cuticular tympanum, or eardrum, on both sides (Fig 1), medial and lateral. The medial tympanum is by far the larger and is an elongated oval. The tympanum on the lateral side of the tibia is much smaller and is circular. The tympana are thin cuticular membranes with air on both sides (like your eardrum) that vibrate in response to sound waves. Tympana are present in adults of both sexes although only males produce sound.
The hindlegs are modified for jumping and are much larger than the others. They are responsible for the characteristic hopping escape behavior of crickets. The large coxa is fused with the trochanter and the femur is greatly enlarged. The femur contains the muscles that extend and flex the angle between the tibia and femur. Extension of this joint propels the cricket into a jump. The tibia fits into the long groove on the femur when the leg is folded.
Note the heavy setae along the posterior margin of the hind tibia. Look at these setae with higher magnification (about 30X) and note that they articulate with the tibia via a flexible articular membrane. Push on one and observe its mobility.
Wings
The wings are evaginations, or folds, of the cuticle and body wall of the posterior two thoracic segments. Each wing is a double layer of body wall consisting largely of cuticle. The characteristic veins of the wings are thickened cuticular tubes involved primarily in support although they contain nerves, epidermis, circulating blood, and tracheae.
The forewings are relatively heavy and are wing covers to protect the larger but more delicate hindwings. Further, the forewings of males function as stridulating organs for the production of sound. The hindwings are pale and delicate and often chewed by other crickets in a laboratory colony. The flight muscles degenerate shortly after the terminal molt and adult house crickets do not fly. Note that the hindwings, if present and complete, are folded accordion-like and are much larger than the forewings.
Lift and spread the forewings and examine them with magnification. Note the heavy longitudinal veins and the abundance of small, branching and anastomosing side veins arising from them.
Stridulating Organ
The adult male stridulating organ consists of a smooth scraper on one forewing that is drawn across a serrated file on the other forewing to produce a species-specific song. The song is important in attracting females and in aggressive encounters with other males. The male adopts a characteristic stance with wings partly elevated when singing and this behavior can usually be observed in the laboratory cricket colony. The modifications necessary for stridulation result in considerable sexual dimorphism in forewing venation.
The male forewing is divided into a dorsal horizontal portion and a lateral vertical portion. Look at the dorsal horizontal surface of the forewing and note the pattern of veins in it. Find the conspicuous curved transverse vein that crosses the wing about one fourth of its (the wing's) length posterior to its anterior end. This vein curves anteriorly at its lateral end. The ventral surface of this vein is the file. Open the wing and twist it so you can focus on the lower (ventral) surface. Look at the file (the large, curved, transverse vein) with high power and you will see its toothed file-like structure. Only the transverse portion of this vein is file-like. The rest is smooth.
The scraper is the smooth longitudinal ridge separating two sides of each forewing. A file and scraper are present on both male forewings.
A large drumhead-like area of the forewing, the harp, vibrates to amplify the sound produced by the file and scraper.
" Use fine scissors to remove the forewing from one side of your male specimen and place it ventral side up on a slide without coverslip or water. Look at the curved transverse vein with the compound microscope. Find the file with the scanning lens and then examine it closely with 100X.
The patterns of venation of males and females forewings are quite different. Use both specimens to compare forewing venation of the two sexes. File, scraper, and harp are absent in the female.
If you are studying an immature cricket, the wings will be absent or represented by small wing pads whose size depends on the maturity of the nymph. Wing pads increase in size with each molt and become wings with the final molt.
Abdomen
" Use fine scissors to cut across the base of each wing to remove them and reveal the abdomen. The cricket abdomen, like that of most insects, consists of 11 segments. It extends posteriorly from the thorax and is by far the largest of the tagmata (Fig 1, 21-1C,F). Most of its segments have no appendages.
Look first at the dorsal midline of the abdomen. The cuticle is transparent in this region and the dorsal tubular heart is faintly visible through it. If your animal is alive, you can see peristaltic waves passing along the heart. If the heart is not beating it will not be recognizable.
With the exception of those at the posterior end, the abdominal segments are easily recognized and counted (Fig 1, 21-1F). Numbered from anterior to posterior, abdominal segments 1-7 are similar to each other and are typical, unspecialized insect segments. They are the pregenital segments and none bears appendages. Segments 8-9 are reduced and modified and may bear appendages. They are the genital segments and their appendages are the external genitalia. The gonopore is on segment 8 but it is hidden by the genitalia. Segments 10-11 are the postgenital segments. Their appendages are sensory.
Look at any of the pregenital segments (2-7) except the first (Fig 1, 21-1F). As typical arthropod segments each is covered by a large dorsal tergite, a ventral sternite, and a pale, softpleurite on each side. Segments 1-8 each have a spiracle in the middle of its pleurites. Look at a spiracle with high power and use a microneedle to open it.
Female
The female external genitalia are the appendages of segments 8 and 9 (Fig 7, 21-11B). Together they form a tubular ovipositor that extends posteriorly from the abdomen and is used by the female to inject eggs into the soil. The ovipositor is a hollow tube formed of right and left appendages but the two sides are not fused together and can be pushed apart with a microneedle to reveal the hollow interior.
During oviposition, eggs exit the gonopore on segment 8, enter the base of the ovipositor, and pass through its lumen (assisted by another derivative of the appendages of segment 9) and are deposited singly in the soil.
The long, sensory, antenniform cerci (singular: cercus) are the appendages of the 11 th segment. The anus is on segment 11 and is
covered by three movable plates derived from the sclerites of the segment (Fig 7, 21-11B). The epiproct lies dorsal to the anus and the two paraprocts flank it. Hold the cricket on its head so you can focus on the anus and use a microneedle to demonstrate the presence of the anus and show how the three plates close over it.
Male
Tergites 9 and 10 are fused as in females but segment 8 resembles the anterior abdominal segments has an unmodified sternite (Fig 8, 21-12B).
Figure 7. Lateral view of the posterior abdomen of a female Acheta. Abdominal sclerites are numbered, T = tergite, P = pleurite, S = sternite. Orthop14L.gif
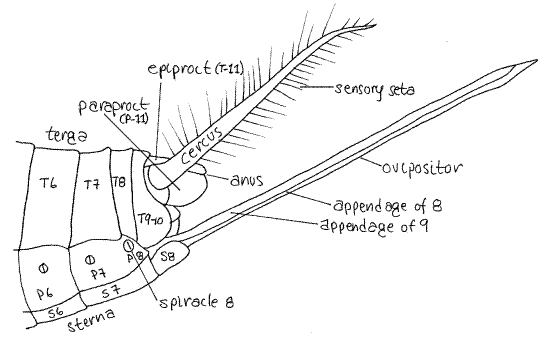
Figure 8. Lateral view of the posterior abdomen of a male Acheta. Abdominal sclerites are numbered, T = tergite, P = pleurite, S = sternite. Orthop15La.gif
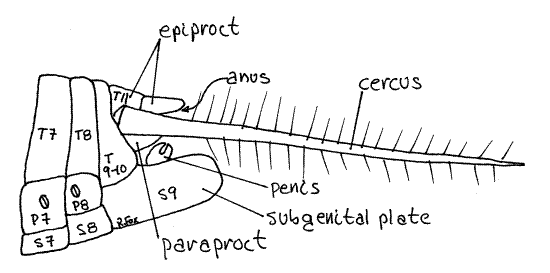
The sternite of segment 9 is a large boat-shaped subgenital plate forming a cup to enclose the male intromittent organ which is the cuticularized, dark penis, or aedeagus. Stand the cricket on its head so the posterior end points toward you and focus on the region. The male genitalia, including an aedeagus, are located in the recess dorsal to the subgenital plate. The genitalia transfer spermatophores to the female.
Segment 11 of males is similar to that of females and has a dorsal anus covered by three movable plates, the epiproct and two paraprocts (Fig 8, 21-12B).
Internal Anatomy
Select one of your crickets for a study of internal anatomy. The dissection should be conducted with the specimen immersed in Woodring’s cricket solution on the stage of a dissecting microscope. Tapwater can be used for a simple study of anatomy of a dead animal but if you anticipate physiological or histological studies or wish the animal to remain alive during the dissection, you should use Woodring’s isosmotic solution.
" Place enough Woodring’s solution in a small dissecting pan to cover the insect. Orient the animal in the pan with its dorsal side up, facing you. Using your fine scissors, make a dorsal longitudinal incision through the body wall of the abdomen. To avoid the middorsal heart, the incision should be a little to one side of the midline. To start the incision, hold the cricket with one hand and gently slip the pointed blade of the scissors under the posterior edge of an abdominal tergite so it penetrates the articular membrane between two tergites. Cut anteriorly through successive tergites keeping the incision shallow to avoid damage to internal organs. Extend the incision anteriorly and posteriorly for the length of the abdomen and pin the cut edges of the body wall aside using #1 stainless steel insect pins. Insert the pins at 45 ° angles into the wax. Extend the incision anteriorly through the thorax, cutting through the thick muscles to reveal the internal organs. Continue the incision to the head but do not cut into the head capsule. Pull the walls of the thorax aside and pin them.
Preview
Examine the interior of the body and identify some major structures to serve as landmarks (Fig 9). The large space you have opened is the hemocoel of the hemal system. It is lined by the diffuse, bright white fat body.
If your specimen is a mature, gravid female, you will see two large, swollen sacs filled with eggs, one on each side of the abdominal hemocoel. These are the ovaries. The eggs are large, elongate ovoids, shaped like bananas or sausages. The size of the ovaries varies depending on the reproductive condition of the female but they may be large enough to obscure your view of other organs. In mature males the testes are large, irregular, white blocks of tissue located laterally in the anterior and middle abdomen. Remove one of the gonads to improve your view of the remaining viscera.
The tubular gut runs longitudinally through the hemocoel from mouth to anus and passes between the two gonads. If the animal is alive, you should see peristaltic waves of muscle contractions in the gut walls. Identify its largest and most obvious region, the crop. This is a large swollen sac in the middle and posterior thorax. (Use the position of the legs to identify regions of the thorax.) You will see a spaghetti-like mass of fine, white or pale yellow tubules in the hemocoel of the posterior abdomen. These are the excretory Malpighian tubules. In living specimens they are in constant writhing motion.
The air-filled trachea of the respiratory system can be seen extending throughout the hemocoel to supply all its organs with oxygen. They branch frequently and are of many sizes. They may appear silver due to the air visible through their thin walls. Much of the space in the thorax is filled with the muscles that operate the legs and wings.
You are now ready to conduct a more careful examination of the hemocoel and its organ systems. The systems are considered in the order in which they are most conveniently exposed.
Body Wall
The cricket body wall is typical of arthropods and includes the epidermis and cuticle. Inside the epidermis are numerous individual muscles associated with the body wall but they are not arranged in distinct circular and longitudinal layers. There is no mesothelium or peritoneum since the body cavity is a hemocoel, not a coelom.
Hemal System
Crickets are a little too small for a study of the hemal system and only the hemocoel is readily visible. The cricket hemal system is similar to that of other arthropods and consists of a tubular dorsal blood vessel and a spacious hemocoel filled with blood. The hemocoel is the functional body cavity and contains the viscera, which are bathed in blood.
Look for the dorsal blood vessel (heart and aorta) in the dorsal hemocoel just inside the body wall on the dorsal midline. It is often destroyed by the initial incision and you may not find it. The abdominal end of the dorsal blood vessel is contractile and is the heart whereas the anterior end is an aorta that opens into the hemocoel. You may have seen the heart beating before you opened the insect. Contractions of the heart force blood anteriorly in the aorta. Paired lateral blood vessels exit the aorta and deliver blood to the hemocoel. Blood in the hemocoel returns to the heart via ostia in its walls.
Respiratory System
The insect respiratory system is independent of the hemal system and oxygen is delivered directly to the tissues without mediation of the blood. Gasses are transported between the spiracles and tissues by an elaborate system of tubular tracheae and tracheoles. The tracheae begin at the spiracles and branch repeatedly, eventually becoming small, delicate tracheoles which extend to, and into, individual cells.
The ancestral tracheate had a pair of spiracles and tracheae for each segment and the pattern in crickets approximates this plan except that a few segments lack spiracles. These segments must be supplied with oxygen by tracheae arising at spiracles in other segments and a network of longitudinal and transverse tracheae ties all the spiracles and segments together into one integrated system. The ventilating current is generated by contraction of abdominal muscles.
Unpin the body wall on one side of the abdomen so you can see the external surface again. Relocate the spiracles on the abdominal pleurites and then look at the inside of the body wall and find their corresponding internal positions. There are two thoracic and eight abdominal pairs of spiracles. Internally each spiracle connects with the complex network of tracheae that extend throughout the body (Fig 21-B,C). Follow some of these tracheae to their target organs (without damaging the organs) and note that some of them extend into the head, where there are no spiracles. A one-way flow of air through parts of the system has been demonstrated with air entering the anterior spiracles and exiting posteriorly.
Being epidermal invaginations, tracheae are lined with cuticle. In most places the cuticle contains a tightly wound, chitinous helix, the taenidia, which supports the trachea and holds it open so air can flow freely through it.
>1a. Remove a piece of trachea and place it in a drop of 0.25% acidified acid fuchsin on a slide. Cover the slide with an inverted culture dish to retard evaporation. Let the preparation sit for an hour. Renew the stain as necessary to prevent the trachea from drying. Remove the stain with a tissue and make a wetmount with tapwater. Examine the slide with the compound microscope and note the composition of the tracheal walls. Find the chitinous taenidia. Its coils may be so tightly wound that they appear to be closely spaced rings rather than turns of a helix.<
Figure 9. Dorsal dissection of the thorax and abdomen of a female Acheta. The left oviduct has been removed and the gut displaced to the left to reveal the nerve cord. Trachea have been omitted. The thoracic and abdominal ganglia are numbered (T = thoracic, A = abdominal). Orthop16La.gif
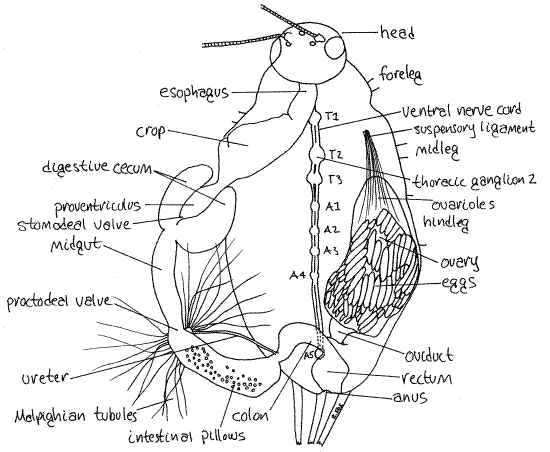
Digestive System
If you have not already done so, remove the gonad and gonoduct from one side, leaving the other side intact for later study. The gut is a large, regionally specialized tube running more or less along the midline in the hemocoel (Fig 9, 21-8A). In insects it consists of an anterior ectodermal foregut (mouth, pharynx, esophagus, crop and proventriculus), middle endodermal midgut (midgut, digestive ceca), and posterior ectodermal hindgut (intestine, colon, rectum, anus).
The mouth opens into the pharynx which extends vertically up into the head capsule (Fig 21-7). The pharynx is entirely contained within the head capsule and cannot be seen. Within the capsule the pharynx opens into the esophagus, which turns posteriorly and can be seen exiting the head to enter the thorax (Fig 9). In the posterior thorax the esophagus widens to become the wide, oval crop where food is stored and some hydrolysis occurs.
The two amorphous white salivary glands on the floor of the hemocoel of the thorax are composed of many lobes. The glands resemble the fat body but are dull grayish-white rather than bright white. They empty via a common salivary duct into the salivarium of the preoral cavity.
The salivary duct resembles a trachea in appearance and structure. The duct is an invagination of the epidermis and its walls are strengthened by chitinous coils as are those of the trachea. The salivary glands probably produce amylase and perhaps other enzymes as well. In some insects, but not crickets, the salivary glands produce silk. As you look for the salivary glands notice the large muscles in the thorax and their elaborate tracheal air supply in the floor and walls of the thorax.
Posteriorly the crop narrows abruptly to become the muscular proventriculus (= gizzard) (Fig 9, 21-8A,B). The proventriculus is a grinding organ whose interior walls are bear tiny, cuticular teeth. Note the numerous large tracheae supplying the musculature of the proventriculus.
" Use fine scissors to open the proventriculus and examine these teeth with high power of the dissecting microscope. Use a jet of water from a squirt bottle or plastic pipet to flush the contents out of the lumen so you can see the teeth.
The pharynx, esophagus, crop, and proventriculus, as part of the foregut, are ectodermal derivatives, and are lined with exoskeleton.
Posterior to the proventriculus is the midgut (Fig 9, 21-8A). It is the absorptive region of the gut and, being endodermal, is not lined with cuticle. The short, thin-walled midgut is hidden by the mass of Malpighian tubules, which must be moved aside. Use your fine forceps to untangle the tubules from the other organs and pull them posteriorly and to the side. Cut any intervening tracheae.
The foregut and midgut are separated by the stomodeal valve (Fig 9, 21-8B). The extreme anterior end of the midgut bears two large, lateral, ovoid digestive ceca (Fig 9). The ceca extend anteriorly to like parentheses ((0)) beside the proventriculus. The hollow diverticula release digestive enzymes into the midgut. The midgut extends posteriorly a short distance from the proventriculus and then joins the hindgut.
The midgut epithelium secretes a permeable chitin-protein peritrophic membrane which encloses the food mass (Fig 21-9). You may later want to open the midgut to see the peritrophic membrane.
The hindgut begins at the posterior end of the midgut and consists of intestine, colon, and rectum (Fig 9). The midgut and hindgut are separated by the proctodeal valve.
Like the foregut, the hindgut is ectodermal and lined with cuticle. The intestine is its anteriormost region and follows immediately after the midgut and proctodeal valve. It makes a long curve to the left and then comes back to the right to the midline. Its epithelium is characterized by conspicuous circular white patches known as pillows, which are covered with masses of symbiotic bacteria that aid in digestion.
The posterior end of the intestine curves back to the midline where it decreases in diameter and becomes the colon (Fig 9). The colon then joins the rectum. The short, wide, muscularrectum empties to the exterior through the anus. The cuticle lining the rectum is permeable to water and other small molecules and this is an area of active reabsorption of water and other valuable materials. Feces is dehydrated and fecal pellets formed in the rectum. Fecal pellets have the shape and markings of the walls of the rectum. So characteristic is the shape and ornamentation of fecal pellets they are routinely used by public health biologists to identify the insects, such as cockroaches, that made them.
The fat body covers most of the inner surface of the body wall and lines the hemocoel. It has many of the functions of annelid chlorogogen and the vertebrate liver and is primarily a storage organ where reserves of lipids and carbohydrates are maintained but it seems also to be involved in nitrogen metabolism and excretion. Almost all of the bright white lining of the hemocoel is fat body. Female crickets mobilize the reserves stored in the fat body during the manufacture of eggs and consequently the fat bodies of gravid females are smaller than those of other crickets.
>1b. With fine scissors open the midgut with a longitudinal incision and find the transparent peritrophic membrane surrounding the gut contents. <
Excretory System
In insects excretion of nitrogenous wastes is accomplished by diverticula of the gut known as Malpighian tubules. In crickets the abundant white or yellow Malpighian tubules are located in a cluster in the middle of the abdomen (Fig 9, 21-8A). Crickets are unusual in that the tubules do not open individually into the gut rather empty into a single ureter which extends posteriorly to enter the gut near the junction of the intestine and colon, well posterior to the junction of the midgut and hindgut. Crickets are the only insects in which the Malpighian tubules do not arise at, or near, the midgut-hindgut junction.
With high power you may be able to see bright white crystals of uric acid in the lumina of the tubules and the ureter. The ureter may be difficult to demonstrate unless it is filled with uric acid. It opens into the gut well posterior to the position of the Malpighian tubules themselves. The yellowish color sometimes exhibited by the tubules is due to riboflavin.
Uric acid is the end product of nitrogen metabolism. Malpighian tubules are secretory kidneys that selectively absorb ions and molecules, including uric acid, from the blood which bathes them (Fig 21-9). These materials are moved along the tubule, to the ureter, and then into the intestine from which they are eliminated with the feces. The walls of the tubules contain visceral muscles and in living specimens the tubules are mobile and active. This motion helps maintain a high concentration of uric acid beside the tubules.
>1c. One of the molecules absorbed by the Malpighian tubules is the dye, amaranth. Place a few drops of a 0.1% solution of amaranth in Woodring’s saline solution on the mass of tubules in the hemocoel of your dissected cricket. Be sure the dye remains in the vicinity of the tubules so they are surrounded by it. Within a few minutes the red pigment can be seen accumulating in the lumina of the tubules. Soon afterwards it can be seen in the ureter, making that tube much easier to visualize. <
Reproductive System
" Make a transverse cut across the rectum, cut the tracheal and other connections between the gut and body wall for the entire length of the gut, and move the gut aside so you have a clear view of the reproductive system and ventral body wall. When finished with your specimen, demonstrate its reproductive anatomy to a classmate who dissected the opposite sex and ask for the same in return.
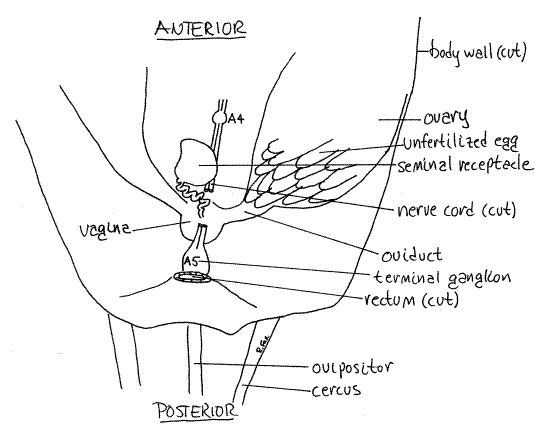
Female
The reproductive system is paired and symmetrical. Its most conspicuous features are the ovaries, of which there is one on either side of the hemocoel (Figs 9, 10). Mature ovaries are large, transparent, and packed with eggs. Each ovary is a bundle of tubular ovarioles composed of oogonia and follicle cells . The upper end of each ovariole is a germarium where oogonia undergo mitotic divisions to produce oocytes and nurse cells. The oocytes then undergo oogenesis (meiosis) to become ova. Nurse cells synthesize RNA and ribosomes which are transferred to the developing ova. Each oocyte is surrounded by follicle cells which transfer yolk from the fat body to the developing oocyte.
Cut or tear the ovary wall and inspect the ovarioles. The large, yolky, banana-shaped eggs are stored in the expanded lower region of the ovary. Eggs in the ovary have not been fertilized.
Posteriorly the short, narrow oviduct exits the ovary and extends medially to join the vagina (= bursa), a translucent, white, spherical organ on the floor of the posterior hemocoel (Fig 10). It lies immediately dorsal to the base of the ovipositor. The vagina opens to the exterior via the female gonopore on abdominal segment 8 at the base of the ovipositor.
A conspicuous, nearly spherical, opaque, white seminal receptacle lies on the floor of the hemocoel anterior to and dorsal to the vagina, to which it is connected by an inconspicuous, convoluted duct (Fig 10). The male intromits sperm into the vagina from which they are moved to the receptacle for storage. During oviposition, eggs pass through the oviduct to the vagina where they are fertilized by sperm from the seminal receptacle. They then move through the ovipositor into the soil.
Male
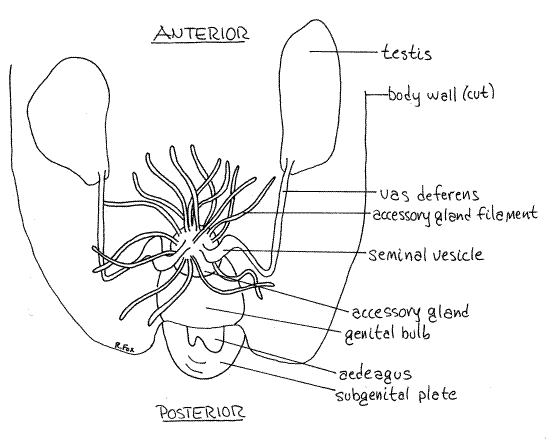
The two testes of Acheta lie laterally in the anterior abdomen (Fig 11, 21-12A). They are large, white, irregularly shaped organs fitting snugly around the proventriculus, digestive ceca, and anterior midgut. The conspicuous accessory gland is a large mass of short, tangled tubules in the center of the posterior abdomen. These tubules resemble Malpighian tubules but are larger in diameter and are always white, never yellow.
The accessory gland connects via a median duct with the large genital bulb which lies ventral to it. The dark, chitinous male external genital organ, the penis (= aedeagus), is attached to the ventral aspect of the bulb and lies in the boat-shaped concavity of the subgenital plate.
Figure 10. Female reproductive system of Acheta. Orthop17La.gif
A very slender, easily overlooked, threadlike vas deferens exits the posterior end of each testis and extends posteromedially across the floor of the hemocoel. Upon reaching a position opposite the genital bulb the vas deferens turns sharply medially and enters the tangle of accessory gland tubules. Within this tangle it expands to form a seminal vesicle for the storage of sperm. The seminal vesicle empties into the lumen of the central region of the accessory gland. The vas deferens is transparent or white and difficult to follow.
The accessory gland consists of a central region from which radiate an abundance of blind tubules of various diameters, lengths, and functions. Together they produce a spermatophore to be transfered to the female during copulation. There is usually a spermatophore present in the lumen of the genital bulb. Sometimes this spermatophore can be extruded from intact, undissected males, by gently squeezing the posterior end of the abdomen.
>1d. Place a seminal vesicle or a spermatophore in a drop of Ringer's solution on a slide. Tease it apart and apply a coverslip. Examine the preparation with 400X and observe the spermatozoa. They are flagellated with elongate heads. <
Reproduction and Development
Sperm are stored in the female seminal receptacle until oviposition, at which time they are used to fertilize eggs passing from the oviduct to the vagina. The female uses the ovipositor to inject up to 200 eggs into the ground where they develop in about three days at 30° C. Development is paurometabolous (Fig 21-13). The eggs hatch into small first instar nymphs, which closely resemble adults except for their small size, lack of wings, and sexual immaturity. Nymphs grow and molt repeatedly, passing through eight nymphal stages, or instars. The ninth, or terminal, molt transforms them into the adult instar, or imago. Insects cease molting after becoming imagos. The imago is sexually mature and has wings, neither of which is true of nymphs.
Figure 11. The male reproductive system. Most of the filaments of the accessory gland have been omitted from the drawing. Orthop18L.gif
Nervous System
The nervous system consists of a dorsal, tripartite brain, or cerebral ganglion, a ganglionated nerve cord, and a peripheral nervous system, including somatic and visceral (somatogastric) components (Fig 9, 21-7). A pair of circumenteric connectives exits the brain and encircles the esophagus to unite ventral to the gut at the subesophageal ganglion. These structures are in the head and will not be seen in this dissection. The ganglionated ventral nerve cord exits the subesophageal ganglion and head and extends the length of the body on the ventral midline of the hemocoel.
" Remove the gut if you have not already done so. If your specimen is a male, remove the accessory glands as well.
The nervous system is visible on the ventral midline but may be obscured by the fat body (Fig 9, 21-8A). If this is the case, remove enough of the fat body to reveal the ventral midline and the nerve cord. This is best accomplished by washing it away with a squeeze bottle of saline.
>1e. The nervous system is white as is the surrounding tissue, and it is difficult to see because of the poor contrast. If you wish, you may stain it to improve its visibility. To do this pour off the fluid in the dissecting pan and place a few drops of 0.5% aqueous methylene blue in the hemocoel. Let it sit for 15 minutes or more, rinse it off, and cover the animal with clean water. The stain will greatly improve the contrast between the nervous system and the surrounding stained tissues. In addition, it will stain the salivary glands deep blue, making it easy to distinguish them from the fat body whose lipids do not stain with methylene blue. <
The double ventral nerve cord extends the length of the thoracic and abdominal hemocoels (Fig 9, 21-8A). The cord is close to the hemocoel in the abdomen but is deeper in the overlying tissue in the thorax. It includes three large segmental thoracic ganglia, one for each thoracic segment but the metathoracic ganglion also includes the ganglia of the first three abdominal segments. Lateral nerves can be seen exiting these ganglia. Five smaller abdominal ganglia are spaced along the nerve cord in the abdomen. The fifth one, the terminal ganglion, is ventral to the rectum and is formed by the fusion of the several ganglia of the posterior abdominal segments (Fig 9, 10). Two large sensory nerves from the cerci enter the posterolateral corners of the terminal ganglion. Lateral nerves exit the ganglia to serve nearby structures.
Sensory System
The major sense organs are the antennae, compound and simple eyes, tympana, and cerci, all of which have been previously discussed.
References
Albrecht FO . 1953. The Anatomy of the Migratory Locust. Althone Press, London. 118p.
Borrer DJ, Triplehorn CA, Johnson NF . 1989. An Introduction to the Study of Insects. Saunders, Philadelphia. 875p.
Brooks WK . 1890. Handbook of Invertebrate Zoology. Bradlee Whidden, Boston. 352p.
Chapman RF. 1998. The insects, Structure and function, 4 th ed. Cambridge Univ. Press, Cambridge. 769 pp.
Comstock JH. 1930. An introduction to entomology. Comstock Pub., Ithaca. 1044 pp.
Comstock JH, Kellog VL . 1916. The Elements of Insect Anatomy. Comstock, Ithaca. 145p.
Gillott C. 1995. Entomology, 2 nd ed. Plenum Press, New York. 798 pp.
Hickman FM. Hickman CP. 1986 . Laboratory Studies in Integrated Zoology. Times Mosby/Mirror, St. Louis. 414 p.
Huber F, Moore T, Loher W (eds.). 1989. Cricket Morphology and Neurobiology. Cornell University Press, Ithaca. 565p.
Miall LC, Denny A . 1886. The Structure and Life-history of the Cockroach (Periplaneta orientalis). Lovell Reeve, London. 224p.
Petrunkevich, A . 1916. Morphology of Invertebrate Types. MacMillan, New York. 263p.
Ross HH. 1965. A textbook of entomology, 3 rd ed. John Wiley & Sons, New York. 539pp.
Ruppert EE, Fox RS, Barnes RB. 2004. Invertebrate Zoology a functional evolutionary approach, 7 th ed. Brooks Cole Thomson, Belmont, California. 963 pp.
Snodgrass RE . 1935. Principles of insect morphology. McGraw-Hill, New York. 667 pp.
Woodring J. Unpublished cricket anatomy and physiology laboratory exercises. Louisiana State University. Baton Rouge.
Supplies
Living or preserved, adult male and female Acheta domestica
Small (anchovy or sardine tin) wax-bottom dissecting pan
Dissecting microscope
Compound microscope
Dissecting set with microdissecting tools
# 1 stainless steel insect pins
Plastic Pasteur pipet
100 ml Woodring’s Cricket solution or 0.7% saline solution
0.5% aqueous methylene blue
8 or 16 oz jars with lids (anesthetizing chambers)
0.25% acidified acid fuchsin
0.1% amaranth in cricket saline
Slides, coverslips
Lab Prep
Living crickets (Acheta) are sold by bait shops but sometimes are not available all year. Acheta can be ordered from Fluker's Cricket Farm, P.O. Box 378, Baton Rouge, Louisiana 70821 (800.735.8537) where they are available year round.
Acheta can be maintained in the laboratory in glass aquaria at or above 30°C, if provided with water and food, such as dry cat or dog food. The aquarium should be kept clean and without sand or paper on the bottom. There should be constant supply of water.
Amaranth is available from Sigma Chemical Co., P.O.Box 14508, St. Louis, Mo. 63178.
Woodring's Cricket Solution: Dissolve 9.1g NaCl, 0.52g KCl, 1.2g CaCl 2•2H 2O, 0.82g MgCl 2•6H 2O in 100 ml distilled water.